To address the challenges facing the theranostic study, Prof. ZHANG Zhijun and his team at Suzhou Institute of Nano-Tech and Nano-Bionics, Chinese Academy of Sciences (SINANO), has developed a series of theranostic nanoplatforms for efficient and simultaneous cancer imaging and therapy.
Iron oxide magnetic nanoparticles (NPs), which are widely used as magnetic resonance imaging contrast agents, drug delivery vehicles and tumor hyperthermia agents, are ideal candidates to build theranostic agents, while VP4, a rotavirus structure protein, could increase the permeability of the intestinal membrane and the cellular uptake of substances. Based on this consideration, ZHANG and co-workers have designed and constructed a VP4-chemically conjugated Fe3O4 NPs for magnetic resonance/fluorescence imaging and chemotherapy (Fig. 1). Their results show that compared with dextran and BSA, coating of VP4 significantly improved the cellular uptake rate of the drug-loaded Fe3O4 NPs, leading to substantially improved cell MRI sensitivity and anticancer effect (Biomaterials, 2012, 33, 7895-7902).
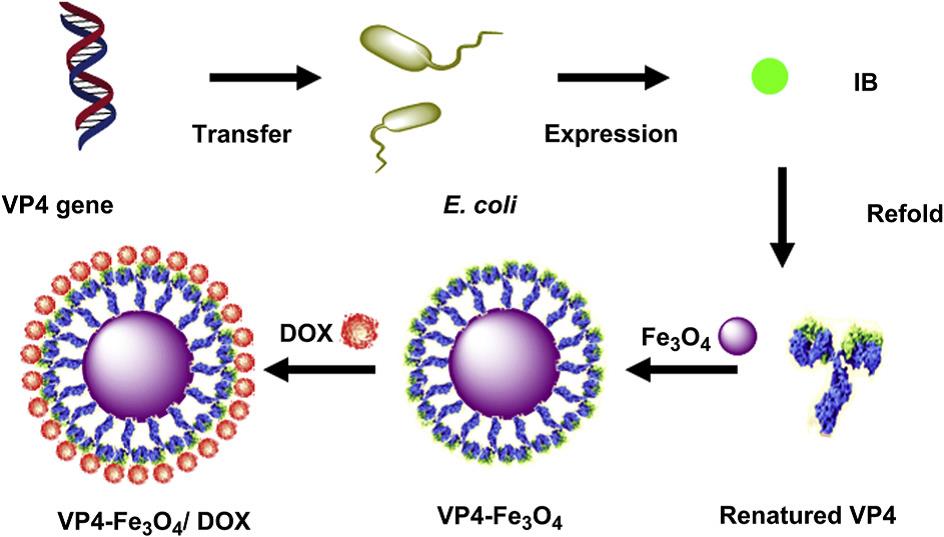
Fig. 1 Schematic diagram showing preparation of rotavirus capsid surface protein VP4-coated Fe3O4 NPs for dual modality magnetic resonance/fluorescence imaging and drug delivery(Image by SINANO).
In another effort to develop new theranostic agents for efficient and safe cancer treatment, the researchers synthesized manganese doped iron oxide NPs modified with denatured bovine serum albumin (MnIO-dBSA). The in vitro experiment revealed that the MnIO NPs exhibited good T1-weighted cellular MRI and photothermal effect under NIR laser irradiation (808 nm). Using 4T1 tumor-bearing mice as an animal model, they further demonstrated that the MnIO-dBSA composites could significantly increase T1 MRI signal intensity at the tumor site (about 2 times) and effectively ablate tumor tissues with photoirradiation (ACS Applied Materials & Interfaces, 2015, 7, 4650-4658).
Recently, the group and collaborators have developed γFe2O3@Au core/shell magnetic gold nanoflowers (MG-NFs)-based theranostic nanoplatform with MRI/PA/SERS multimodal imaging and photothermal therapeutical functionalities. The obtained MG-NFs exhibited synergistically multimodal imaging features including ultrasensitivity from SERS, precise anatomical localization from PA imaging, and good spatial resolution from MR imaging for precise tumor size, localization and boundary identification. Moreover, MG-NFs have excellent photothermal effect that leads to efficient tumor ablation upon photo irradiation (Advanced Materials.2015, 27, 5049-5056).
Compared with the magnetic NPs aforementioned and many other nanomaterials, the two-dimensional nanomaterial graphene oxide (GO) exhibits many distinct advantages, such as ultra-high drug loading ratio, ease in functionalization and scaling up, and low production cost, in the construction of theranostic nanoplatforms. The researchers developed a theranostic agent with dual modality T1-weighted MRI/fluorescence imaging and chemotherapy, by formation of GO-gadolinium complexes (GO-DTPA-Gd), followed by loading with an anticancer drug doxorubicin (DOX) (Fig. 2). Thus-obtained GO-DTPA-Gd complexes exhibit significantly higher r1 relaxivity enhancement than the commercially used Magnevist, being 2.4 times at 11.7 T. Particularly, the GO-DTPA-Gd complexes can be efficiently internalized into cells for T1 MR imaging. Moreover, the DOX-loaded GO-DTPA-Gd system presents significant cytotoxicity against Human hepatocellular carcinoma (HepG2) cells (ACS Applied Materials & Interfaces, 2013, 5, 13325-13332).
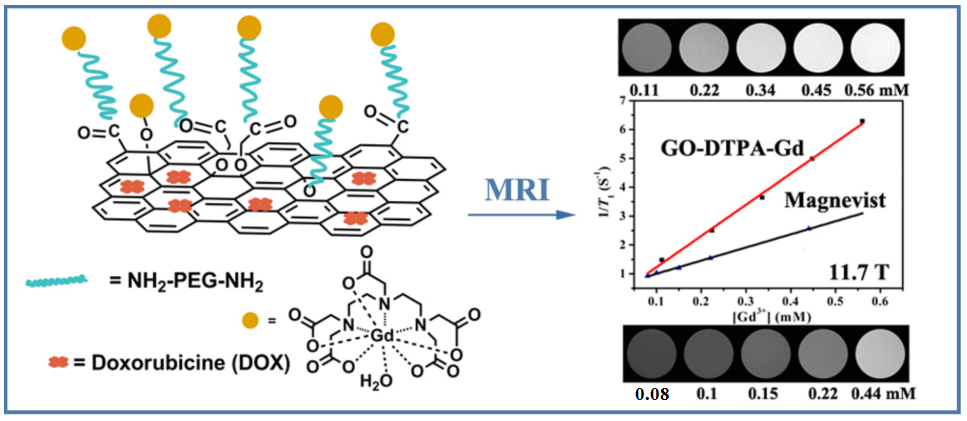
Fig. 2 Schematic illustration of synthesis of the GO-DTPA-Gd complex for MR imaging and drug delivery(Image by SINANO).
Cypate is an organic dye with good near-infrared imaging and photothermal properties. However, the poor photostability severely limits its wide application in biological imaging and photothermal therapy. To address this problem, the research team and collaborators prepared GO-Cypate composites by covalent coupling. Experiments show that the GO-Cypate composites can significantly improve the water solubility, light stability and cellular uptake efficiency of Cypate. Furthermore, GO-Cypate shows good accumulation at tumor site, allowing long time and stable fluorescence imaging and, tumor ablation upon NIR irradiation. The work was published as a frontispiece article in Advanced Functional Materials ,2015, 25, 59-67.
The recent advances in the development of nanotheranostics made by the SINANO team have gained attention from researchers in nanomedicine. Most recently, the research team has published, by invitation, a review article (Small, 2016, DOI: 10.1002/smll.201600635), and a book chapter (World Scientific Publishing, Singapore, 2016, ISBN: 978-9814635417) in the field of nanotheranostics.
The research was supported by the National Natural Science Foundation of China, Ministry of Science and Technology, and Chinese Academy of Sciences.
Contact information:Prof. ZHANG Zhijun
Suzhou Institute of Nano-Tech and Nano-Bionics ,Chinese Academy of Science
Suzhou, Jiangsu 215123, China.
E-mail: zjzhang2007@sinano.ac.cn
downloadFile
