In the past few decades, researchers have never stopped questing contrast agents (CAs) with high resolution and safety to overcome the drawbacks of small-molecule contrast agents in clinic. Compared to X-ray, micro CT and ultrasound imaging techniques, Magnetic resonance imaging (MRI) technique have attracted more and more attention in clinical diagnosis of cancer with high spatial resolution and non-invasive, especially used for early diagnosis of cancer. However, the routinely MRI detection usually can not always distinguish the pathological tissues and normal tissue clearly in clinic. According to statistics, nearly 50% of MRI detection needs the aid of CAs to improve the contrast resolution. The commercial CAs usually consist of individual Gd3+ chelated with a low molecular weight acyclic or cyclic ligand. These small-molecule CAs possess low relaxivity, nonspecificity and short blood residence time, which have been major limitations for their clinical applications.
Recently, PEI Renjun's group has developed several biocompatible Gd-based macromolecular contrast agents (mCAs) for tumor-targeted magnetic resonance imaging.
Polysaccharide-based MRI probes have been widely investigated, especially chitosan and its derivatives. Literatures reported that hydroxyl groups on the glucose ring can attract water molecules, thereby improving the local density of water molecules. This can increase the water exchange rate in Gd molecules and result in enhanced T1 relaxivity. Chitosan, a natural cationic polysaccharide, is considered to be a biodegradable polysaccharide with low toxicity in experimental animals and human, and has been recognized as a good candidate for MR imaging agents. PEI Renjun's group prepared a mCAs based on chitosan derivative. Briefly, Oligoethylenimine (OEI, 800 Da) was grafted to N-maleated chitosan (NMC) via Michael addition, followed by conjugation of Gd-DTPA and Folic acid. The resulting NMC-g-OEI-DTPA-Gd was further functionalized with folic acid, which endued the polymer with the targeting ability. Our results showed that the obtained CA exhibited much higher relaxivity than Gd-DTPA, and produced much brighter images than Gd-DTPA in vitro and in vivo (J. Magn. Reson. Imaging, 2016, 44, 23-29). Furthermore, Poly(aspartic acid) (PAsp) has been employed as the potential backbone for the preparation of different carrier systems, due to its low cytotoxicity, good biodegradability and excellent biocompatibility. Herein, a PEGylated block copolymer was designed as a mCA by PEI Renjun group. The PEGylated block copolymer was prepared by the ring-opening polymerization of β-benzyl-L-aspartate N-carboxy-anhydride (BLA-NCA) initiated by the terminal group of mPEG-NH2, followed by removing the protecting groups and conjugating with oligoethylenimine (Mw=800), Gd-DTPA and folic acid. Our results showed that the obtained mCA exhibited much higher relaxivity than Gd-DTPA, and produced much brighter images both in vitro and in vivo. (J. Mater. Chem. B., 2016, 4, 3324-3330).
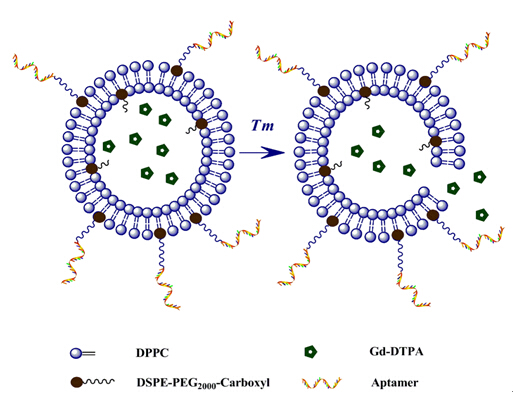
These biodegradable mCAs mentioned above do express excellent tumor-targeting ability and provide a positive signal image, but the degradable behaviors are not under control. PEI Renjun's group offer a new strategy to synthesize a biodegradable contrast agent with excellent comprehensive properties. Briefly, a brush-like mCA composed of SS-PAA with alkynyl groups and azide-terminated oligolysine via click chemistry, was designed and synthesized to increase the loading density of Gd. As reported by previous literatures, disulfide bond has an ability to degrade rapidly under reduced condition. To further polish up the performance, folic acid was grafted onto the polymer to endow this CA with active targeting capacity. Therefore, this biodegradable mCA may have great potential to improve the efficiency and solve the safety concerns impeding the clinical application of mCAs (Bioconjugate Chem., 2016, 27, 151-158).
Among all these carriers, dendrimer, a macromolecule with precise molecular structure, perfectly monodisperse and lots of chemical groups on the surface, can be conveniently functionalized as different platform for drug release, gene delivery and MRI CAs. However, the general procedure for synthesis of dendrimers was usually complicated and waste lots of time with low yield. Hyperbranched polymers then emerged as an alternative to dendrimers. Compared to dendrimers, Hyperbranched polymers can prepared through one-step polymerization reaction with a high yield. PEI Renjun group fabricated a hyperbranched polylysine via one-step polycondensation of L-lysine, followed by conjugation of DTPA-Gd and targeting molecule. The FA-HBPLL-DTPA-Gd showed significant signal intensity enhancement in the tumor region at various timepoints and provided long time window for MR examination. (Biomacromolecules, 2016, 17, 2302-2308)
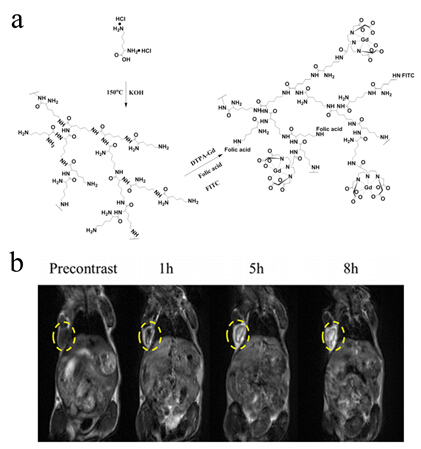
Figure 1 Synthesis scheme of Gd(III)-based hyperbranched polylysine.(Image by SINANO)
downloadFile