Rapidly growing demands for electrochemical energy storages are driving scientists around the world to develop next-generation high energy density lithium battery. Lithium sulfur (Li-S) battery, based on reversible redox reactions between sulfur cathode and lithium metal anode, promises a theoretical energy density as high as 2600 Wh/Kg, therefore, has great potential in applications such as EVs, HEVs and portable electronic products. However, the short cycle life induced by the shuttle effect of the soluble polysulfide will hamper the commercialization of Li-S batteries.
Recently, professor CHEN Liwei’s group in the Suzhou Institute of Nano-Tech and Nano-Bionics (SINANO), Chinese Academy of Sciences, has made progresses in developing an unconventional wrapping method to block the shuttle effect in Li-S batteries.
Traditional sulfur wrapping strategy typically involves the preparation of carbon/sulfur composite particles with a wrapping layer and the wrapped C/S composite is assembled into coin cells for testing. A dilemma exists in designing the wrapping layer when using this approach, as illustrated in Figure. 1b. If the preassembly wrapping layer was designed to be perfectly compact and tight (to completely block the polysulfide diffusion), the electrolyte would not infiltrate the C/S composite in the assembled cell. Then, the battery will exhibit poor performance. Alternatively, if the preassembly wrapping layer was imperfectly designed with pores and/or cracks that allow for the penetration of the electrolyte into the C/S composite, solvated polysulfides can also leak out of the wrapping layer via these defects, which can lead to improved but diminishing performance (Figure. 1c). The latter scenario represents most sulfur wrapping work reported to date.
In this new work, C/S composite particles are imperfectly coated with a wrapping layer, and the material is assembled into coin cells. The imperfect wrapping layer allows the infiltration of adequate electrolyte into the C/S composite particles. Significantly, a special functional additive is added to the electrolyte to react with the initially imperfect wrapping layer to form a second wrapping layer after the battery is assembled. The second, post-assembled and in situ-formed wrapping layer is designed to be compact and tight to completely block the shuttle effect, which allows polysulfide dissolution into the electrolyte within the interior of the wrapped composite particles (Figure. 1d).
This in situ wrapping approach solves this trade-off by first allowing the electrolyte to infuse into the interior of the porous cathode material and then closing the pores by a surface reaction with a special additive in the electrolyte. The research results show greatly improved Coulombic efficiency and cycle life of lithium sulfur batteries by this in situ wrapping approach. Importantly, the capacity decay of the cell at 1000 cycles is as small as 0.030% per cycle at 1C. This work has published in Nature Communications.
This research work is supported by the “Strategic Priority Research Program” of the CAS, the Ministry of Science and Technology of China, and Natural Science Foundation of China.
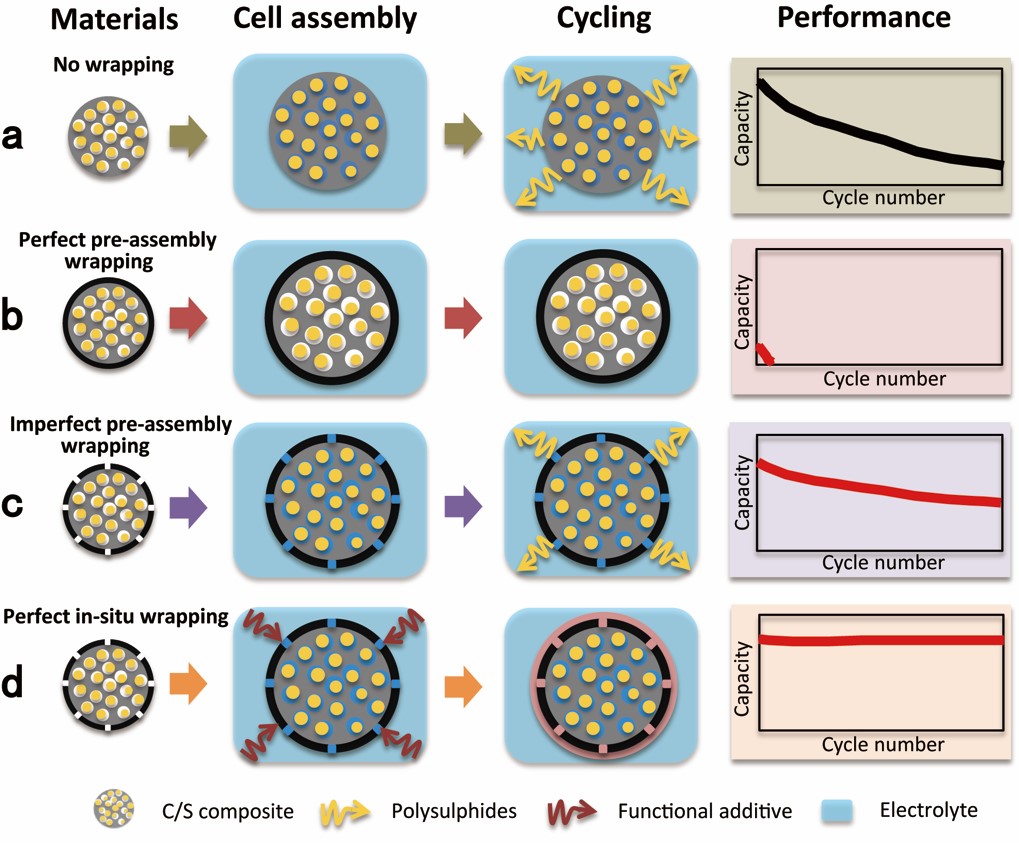
Fig.1. Schematic illustration of the unique in situ wrapping strategy for Li-S cathode structures. (a) The no wrapping case, exhibiting severe capacity decay in cycling. (b) Perfect wrapping of C/S materials before battery assembly, exhibiting poor coverall performance due to lack of electrolyte in the active material. (c) Imperfect wrapping of the cathode material before battery assembly, exhibiting improved cycle stability than the no wrapping case. (d) Perfect post-assembly in situ wrapping of the cathode material, exhibiting ideal cycle stability by blocking polysulfide shuttle while allowing for electrolyte infiltration in the active material.(Image by SINANO)
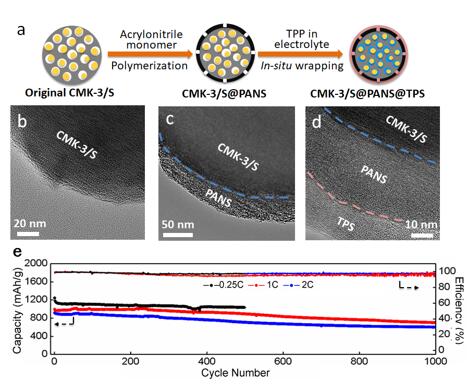
Fig. 2. (a) Schematic illustration of the in situ wrapping process. TEM images of (b) CMK-3/S, (c) CMK-3/S@PANS and (d) CMK-3/S@PANS@TPS particles. (e) Long cycling performance of cells with CMK-3/S@PANS@TPS cathodes at 0.25C, 1C and 2C.(Image by SINANO)
Reference:
https://www.nature.com/articles/s41467-017-00656-8
Contact Information:
Prof. CHEN Liwei, Suzhou Institute of Nano-Tech and Nano-Bionics, Chinese Academy of Sciences
Email: lwchen2008@sinano.ac.cn
downloadFile
