Spinal cord injury (SCI) often results in loss of neurons and axonal retraction at the injury site, interrupting the neuronal relays such that information can no longer be effectively transmitted through the injury, ultimately resulting in functional deficits of sensation and movement below the injured site. The main challenge of SCI repair is the adverse neural regeneration microenvironment.
Previous studies have demonstrated that myelin-associated inhibitors (MAIs) and CSPGs inhibit neural regeneration by activating epidermal growth factor receptor (EGFR) signals. Treatment with EGFR neutralizing factors in animals after SCI was reported to lead to enhanced neural regeneration and behavioral restoration. Dai Jianwu's research group previously employed scaffold-based EGFR antibody cetuximab delivery to attenuate the inhibitory effects of inhibitors and rehabbed a neuronal differentiation microenvironment for SCI treatment, with induction of neuronal differentiation of NSCs. In addition, microtubules are the main structure that constitutes the cytoskeleton, and high microtubule stability is the basis for neurite elongation and growth. Studies have found that the microtubule stabilizer taxol can inhibit scarring in the lesion site and promote axonal regeneration.
However, if only the neurons lost in the injured area are supplemented and the axons of the injured stump are not completely regenerated, the newborn neurons will not be able to form sufficient targets with the host neurons; if only the axons regenerate into the damaged area and rare neurons in the damaged area do the bridging, it is difficult for the regenerating axons to cross the long-distance injury to go through the neural pathway.
Recently, a research team from Suzhou Institute of Nano-Tech and Nano-Bionics, Chinese Academy of Sciences successfully combined the EGFR antibody cetuximab and the microtubule stabilizing molecule taxol into a self-developed neuroregen scaffold to evaluate the treatment effect for severe SCI(Figure 1). Among them, EGFR antibody cetuximab can promote neuronal differentiation by blocking EGFR signal; microtubule stabilizing molecule taxol can promote axon growth by regulating microtubule stability and reduce scar formation in injured area; the neuroregen scaffold can guide the directional regeneration of the nerve, reduce the scar deposition by physical occupation, and serve as a drug delivery carrier.
The results showed that animals transplanted with the combined scaffolds had significantly increased neurogenesis in the injured area compared with the control group, the material group and the single-factor scaffold group, and exhibited the myelination of nerve fibers and functional neuron production. Furthermore, cetuximab, taxol and scaffolds showed different degrees of inhibition on different components of scars, and multiple scar components were deposited least in the injured area of the combined treatment group(Figure 1). Finally, the functional recovery of hind limbs of SCI animals was best after combined treatment(Figure 2). The combined functional biomaterials developed in this study have a good application prospect for enhancing SCI repair, and provide theoretical support for future clinical transformation.
This work entitled "Cetuximab and Taxol co-modified collagen scaffolds show combination effects for the repair of acute spinal cord injury" was recently published in Biomaterials Science (Cover article).
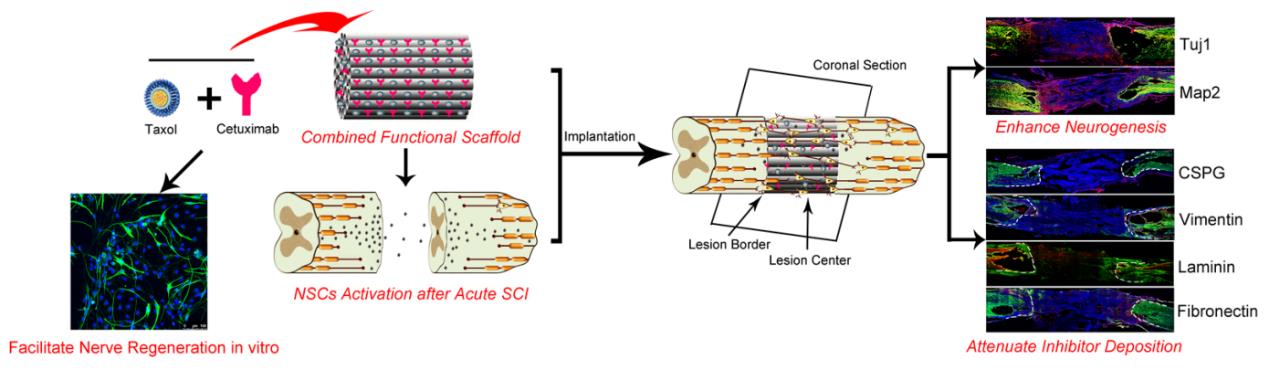
Figure1.The combined functional scaffold transplantation can effectively improve the recovery of hind limb function in rats with spinal cord transection. (Image by SINANO)
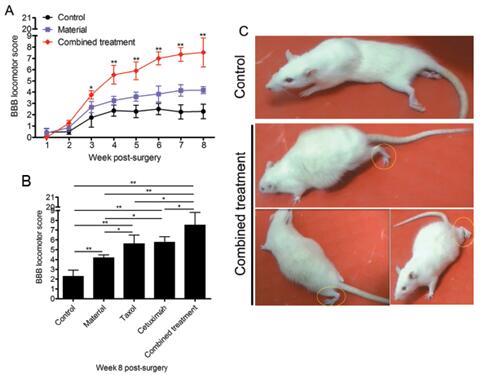
Figure 2 The combined functional scaffold transplantation can effectively improve the recovery of hind limb function in rats with spinal cord transection.(Image by SINANO)
Reference: https://pubs.rsc.org/en/content/articlelanding/2018/bm/c8bm00363g#!divAbstract
Contact Information:
Prof. DAI Jianwu, Suzhou institute of Nano-Tech and Nano-Bionic, Chinese Academy of Sciences; Institute of Genetics and Developmental Biology, Chinese Academy of Sciences.
Email: jwdai@genetics.ac.cn
downloadFile
