Since the first rechargeable lithium ion battery (LIB) was commercialized by SONY in 1990s, LIB becomes one of the most favorite energy storage devices for upcoming mobile electric devices and hybrid vehicles because of their high performance. Recently, in order to meet the industrial and commercial requirement, high-capacity electrochemical active materials based LIB is being vigorously pursued. Unfortunately, this type of materials, always suffer from substantial volume changes during insertion and extraction of Lithium (Li) ion, which causes the collapse of the electrode and shorten the cycle life of cell.
Materials science has evolved over the past decades. However, most of research on electrode for energy storage has been focused on active material itself. It is clear that investigating isolated active materials is no longer sufficient to solve all kinds of technological challenges for the development of modern battery infrastructure. Considerable amounts of attentions should be paid on the entire electrode system where studying the interface between individual components within the system is of paramount importance.
Recently, Prof. JIN Jian’s group at Suzhou Institute of Nano-Tech & Nano-Bionics, Chinese Academy of Sciences (SINANO) report a system-level strategy of designing RGO/SnO2 composites based anode electrode aims at enhancing the energy-storage performance of RGO/SnO2-based materials, especially their cyclic performance. The RGO/SnO2 composite is firstly coated by polydopamine (PD) layer which function as a buffer. A crosslink reaction is then built between buffer and Polyacrylic acid (PAA) binder to integrate individual RGO/SnO2 composites into a whole system through forming a robust network of covalent bond in the anode. These results indicate the effectiveness of system engineering to the final performance of anode electrode, especially its long-term cycle stability. As a result, the designed anode system exhibits an outstanding energy capacity up to 718 mAh/g at current density of 100 mA/g after 200 cycles. (RSC Advance 2013, 3, 1307.) and (Nano Lett. 2013, 13, 1711.)
Furthermore, from the interface chemistry point of view, a system-level strategy of designing PD coated RGO/sulfur composites cathode aims at enhancing the cyclic performance has been achieved very recently. The electrode demonstrated excellent cyclic performance with a discharge capacity of 728 mAh/g after 500 cycles at the current density of 0.5 A/g (a very small capacity loss of 0.41 mAh/g per cycle). Most importantly, 530 mAh/g was obtained even at a higher current density of 1 A/g after 800 cycles. These results indicate the importance of chemically designing interfaces in the whole electrode system on achieving improved performance of electrode of LIB. This work has been recently published in Nano Lett. 2013, DOI:nl4018868
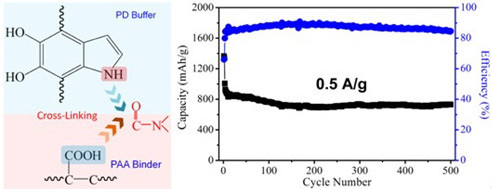
Figure 1. (a) Schematic of the formation of cross-link reaction between buffer and binder in electrode of Li-ion battery. (b) Cyclic performance of Li-S battery. (Image by SINANO)
This work was supported by the National Basic Research Program of China (grant no. 2013CB933000) and the National Natural Science Foundation of China (grant no. 21273270 and 21004076), and the Key Development Project of Chinese Academy of Sciences (grant no. KJZD-EW-M01-3).
downloadFile
