Environment and energy issues are two big challenges for sustainable development of the civil world. The development of clean energy technologies, such as high power lithium battery, wind power, and solar power, has become the main research direction that may revolutionize the future energy industry. With the past decade’s performance improvement and technical innovation, lithium battery occupies an important place in the current energy storage industry. However, the fast development of new electronic devices and electric vehicles put forward much higher requirements for the new-generation lithium batteries.
As an emerging energy storage technology, Li/S battery attracted much attention from the field due to its high theoretical specific energy, low cost, anf environmental friendly features. The theoretical specific capacity and energy density are as high as 1675 mA h g-1 and 2600 Wh kg-1, respectively, which is significantly higher than the current lithium-ion batteries.
Recently, a research group lead by Professor Yuegang Zhang at Suzhou Institute of Nano-tech and Nano-bionics (SINANO), Chinese Academy of Sciences, has made significant progress in development of Li/S battery based on functionalized graphene-sulfur nanocomposite materials. The researchers designed and prepared polyaniline (PANI)-modified cetyltrimethylammonium bromide (CTAB)-grapheme oxde(GO)-sulfur(S) nanocomposits as sulfur cathode materials and significantly enhanced the performance of Li/S battery. The improved performance can be attributed to the lower charge transfer resistance and the alleviated dissolution of polysulfides in the PANI-modified CTAB–GO–S cathodes. The work was published in Nano Research (2014,7, 1355-1363). In order to further improve the electrical conductivity of graphene-sulfur nanocomposite materials and to prevent polysulfide dissolution, they further wrapped the sulfur nanoparticles inside nitrogen-doped graphene sheets (S@NG). The new cathode material significantly improved the rate performance and the cycle-life of the Li/S cells. They show that the Li/S@NG can deliver high specific discharge capacities at high rates, i.e. ~1167 mAh g-1 at 0.2 C, ~1058 mAh g-1 at 0.5 C, ~971 mAh g-1 at 1 C, ~802 mAh g-1 at 2 C, ~606 mAh g-1 at 5 C. The cells also demonstrate an ultra-long cycle life exceeding 2000 cycles and an extremely low capacity-decay rate (0.028% per cycle), which is among the best performance demonstrated so far for Li/S cells. The excellent performance can be attributed to the well-restored C-C lattice and the unique lithium polysulfide binding capability of the N functional groups in the NG sheets. The results were published on Nano Letters(2014, 14, 4821-4827) as a communication article. The group also designed and synthesized another cathode material with nanosized sulfur attached onto densely packed and conductive graphene (G) nanosheets, through the low temperature interfacial synthesis of sulfur in the 3D grapheme hydrogels and a subsequent controllable soft densification process. They discovered that the compact S-G structures is much more efficient in improving the bonding between sulfur and graphene, immobilizing sulfur, and restraining the polysul?de diffusion within the more closed pores than loose counterpart, which enabled improved volumetric discharge capacities, high rate, and long-term cycling performance (Nano Energy,2015,12,468-475).
In addition to the sulfur cathode materials, Zhang’s group also achieved progress in synthesizing other nanomaterials that could be used in lithium ion batteries, supercapacitors, and photo-catalysts. Examples include V2O5 microflowers cathode material for lithium ion battery (J. Mater. Chem. A, 2015, 3, 1103-1109 ), niobium oxide nanosheets cathode material for lithium ion battery (Scientific Reports 2015, 5, 8326), hierarchical 3D carbon nanostructure for high-energy flexible supercapacitors (Chem. Mater., 2015, 27, 1194–1200), and three-dimensional hyperbranched TiO2 nanowire arrays for photoelectrochemical hydrogen production (J. Mater. Chem. A, 2015, 3, 4004-4009).
The work was financially supported by National Natural Science Foundation of China (NSFC), China Postdoctoral Science Foundation, and the Natural Science Foundation of Jiangsu Province, China.
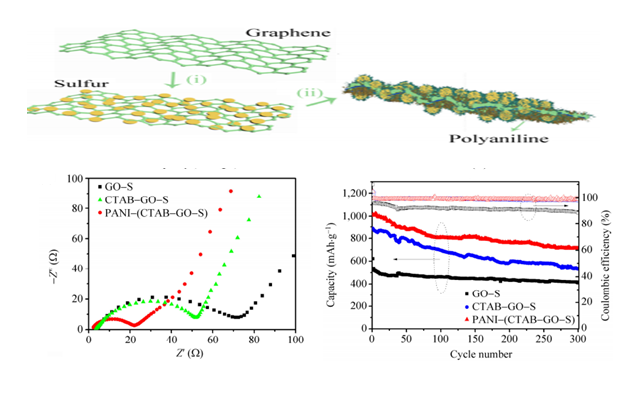
Figure 1. The preparation process and the electrochemical performance of PANI (CTAB–GO–S) nanocomposites (Nano Research, 2014,7,1355-1363)(Image by SINANO)
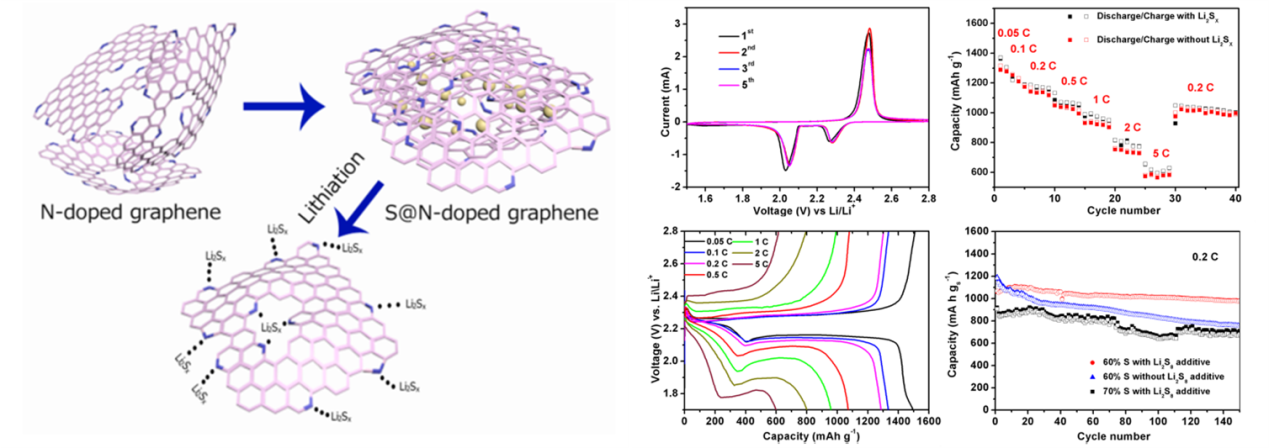
Figure 2. The synthesis route and the electrochemical performance of S@NG nanocomposite (Nano Letters, 2014, 14, 4821-4827)(Image by SINANO)
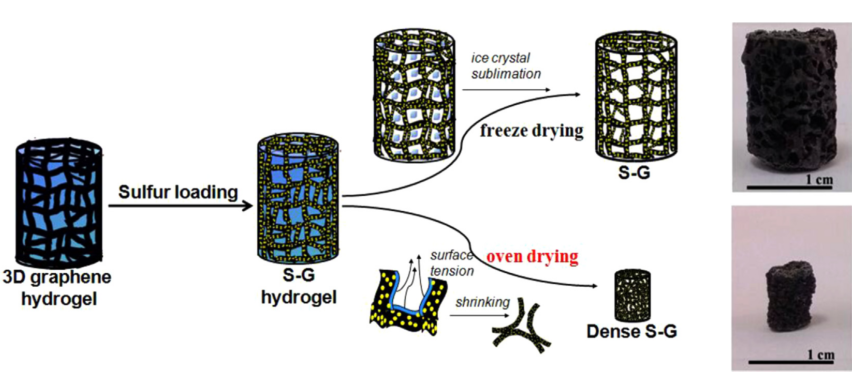
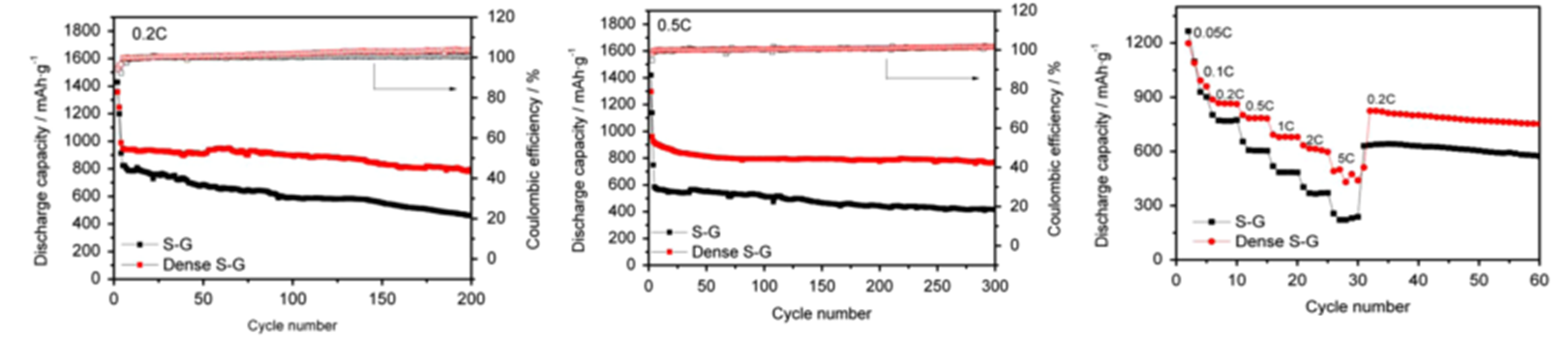
Figure 3. The formation process and the electrochemical performance of S-G and dense S-G (Nano Energy, 2015, 12,468-475)(Image by SINANO)
downloadFile
