Recent research has shown for the first time that reactive free radicals can be generated on carbon nanotubes (CNT) since CNT was discovered 25 years ago. Mediated by CNT free radicals, directly intertube crosslinking of CNTs is realized at room temperature and ambient conditions, which was previously achieved only under extremely high temperature, high pressure, or strong electron/ion beam irradiation conditions.
Recently, the Optoelectronic Interface Laboratory led by Professor CHEN Liwei at Suzhou Institute of Nano-Tech and Nano-Bionics (SINANO), Chinese Academy of Sciences, made a breakthrough in carbon nanotube free radical chemistry and exploited it for low-energy CNT welding. The findings were published in the Oct. 12, 2016 issue of the journal Nano Letters.
"Different from the conventional wisdom that carbon nanotubes are efficient free radical scavengers rather than being reactive radicals themselves, in this work, we found that reactive free radicals can in fact be generated on carbon nanotubes via reductive defluorination of highly fluorinated single-walled carbon nanotubes (FSWNTs)," said Prof. Chen's former PhD student GAO Yunxiang, the leading author of the recent Nano Letter publication who initially proposed using FSWNTs for this study, and was acutely aware of the unusual observations and discovered the reaction mechanism. "Carbon nanotubes can consequently be directly crosslinked, or “welded”, via intertube radical recombination with a surprisingly low energy barrier, under room temperature", he said.
The new CNT radical chemistry can be applied to fabricate strengthened carbon fibers under mild conditions, according to co-first-author CHEN Hongwei, another PhD student supervised by Prof. CHEN Liwei who contributed in capturing the evidences for the existence of CNT radicals and demonstrating their potential applications in enhancing the tensile strength of CNT fibers.
"The capability of handling CNT crosslinking at room temperature could bring a technological leap for the fabrication of strong yet lightweight carbon materials," said Prof. CHEN Liwei, "it is also a powerful new tool for future CNT-based materials design."
Mechanism of the CNT free radical chemistry
The chemistry of carbon nanotubes is important for fundamental sciences as well as practical processing, separation, and application of CNT-based carbon materials. Previous CNT chemistry research involving free radicals always feature in external free radical species reacting with CNTs.
"An opposite class of reactions, where CNT itself is turned into a reactive free radical species, is thought to be in conflicts with traditional understanding of CNTs before the publication of our work", said Prof. Liwei Chen, "because the large π-electron conjugation system in pristine CNTs effectively quenches free radicals."
The newly discovered CNT radical mechanism is illustrated in Figure 1. "The reason we succeed in obtaining reactive free radicals on the CNT backbone is that we exploited the severely disrupted π-electron conjugation systems in fluorinated CNTs. The localized π-conjugation system facilitates the generation of reactive radicals and prevents intra-tube radical recombination that recovers normal CNTs", said Yunxiang Gao. "The reduction of FSWNTs with electron donors leads to the elimination of fluoride ions, followed by the generation of localized free radicals on the CNT lattice. The generated CNT radicals can either initiate polymerization of alkene monomers as traditional organic free radical initiators do, or undergo an inter-tube recombination to form crosslinked CNTs, or say, polyCNTs."
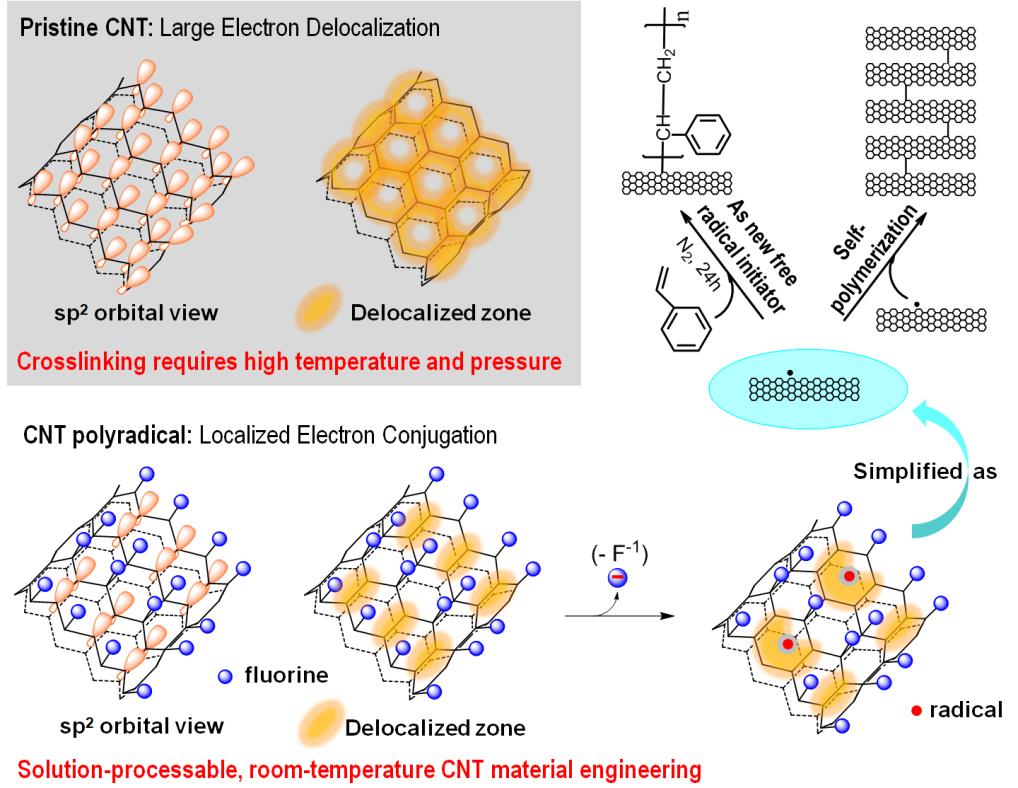
Figure 1. The generation of free radicals on FSWNTs via reductive elimination of fluoride, resulting in CNT polyradicals that are capable of initiating polymerization of alkene monomers or recombinating with other CNT free radicals to form crosslinked CNTs.(Image by SINANO's group)
Application in CNT fiber materials
Individual carbon nanotubes are one of the strongest and stiffest materials man has ever known. However, due to the weak van der Waals interaction between individual CNTs, the extraordinary intrinsic strength of CNTs cannot be carried over to bulk assemblies such as CNT fibers. Crosslinking of CNTs has been a major approach for enhancing mechanical properties of CNT-based materials.
"Previous works indicate that direct inter-tube covalent crosslinking of CNTs requires extreme conditions such as high temperature of ~ 2000 K and high pressures of ~ 35 GPa. With our finding of the new CNT radical chemistry, we make it happen at room temperature and atmosphere pressure", said Prof. Liwei Chen.
Figure 2A-H show the morphology of CNT fibers fabricated using different treatments including reductive defluorination of fluorinated CNT fibers. As shown in Figure 2I, "directly crosslinked CNT fibers via repeated fluorination and defluorination treatments show great improvement in tensile strength", said Hongwei Chen. "It can be concluded that intertube covalent CNT crosslinking introduced by the room-temperature CNT radical recombination changes the intertube interaction in CNT bundles and significantly improves the mechanical property of CNT fibers."
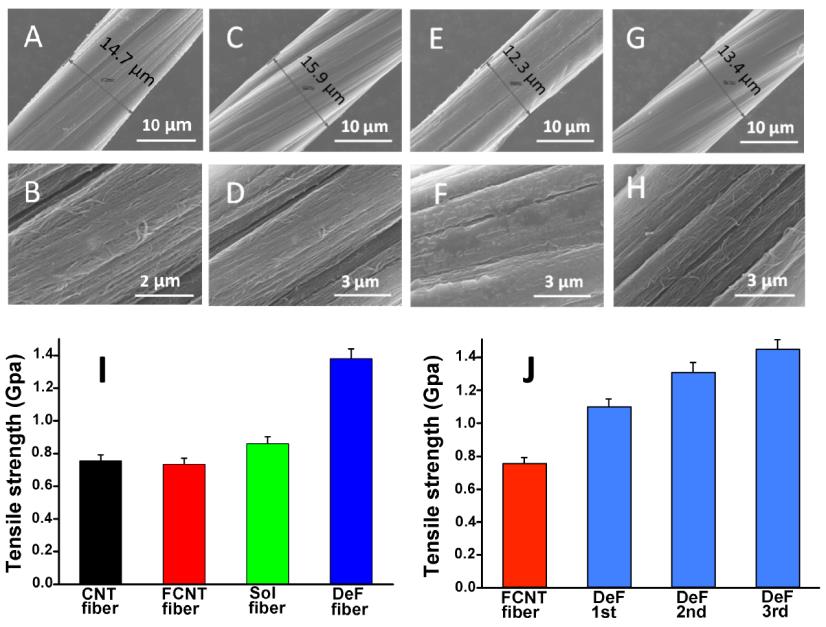
Figure 2. SEM images of (A, B) pristine CNT fiber; (C, D) CNT fiber after fluorinated (F-fiber); (E, F) F-fiber after defluorinated (DeF-fiber); (G, H) Solvent-soaking fiber (Sol-fiber); (I) tensile strength of the fibers with different treatment; and (J) tensile strength of the fibers with repeated fluorination/defluorination.(Image by SINANO's group)
The origin of CNT radical reactivity
The drastically reduced requirement in reaction conditions for covalent CNT crosslinking from tens of Giga Pascal and thousands of Kelvin to ambient environment, is another highlight of this work. According to Prof. Liwei Chen, "the strong electronegativity of fluorine and the high curvature strain of the CNT lattice are the key contributors in the reactivity of CNT radicals."
With the electrochemical cyclic voltammetry study presented in Figure 3A, which compares the irreversible reduction potential of the perfluoro-FSWNTs with that of perfluoro-fullerenes (PF-C60) and perfluorodecalin (PF-decalin), Gao explained that "the higher the molecular backbone curvature, the less negative the reduction potential for C-F bond dissociation, which means the lower the energy barrier for obtaining free radicals."
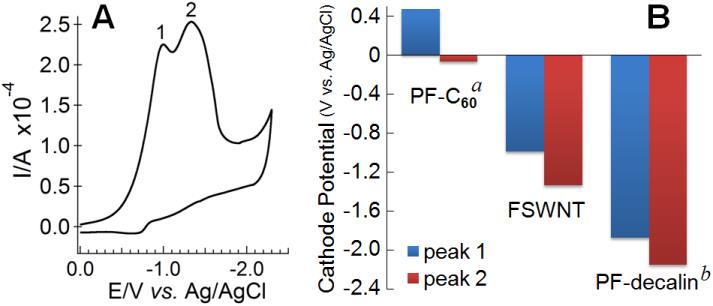
Figure 3. (A) Cyclic voltammogram of a FSWNT membrane on Pt electrode measured in ACN with 0.08 M tetrabutylammonium hexafluorophosphate, scan rate 0.05 V/s. (B) Peak cathode potential for fluorocarbons with different molecular curvature.(Image by SINANO's group)
Journal Reference
Yunxiang Gao, Hongwei Chen, Jun Ge, Jingna Zhao, Qingwen Li, Jianxin Tang, Yi Cui and Liwei Chen Direct Intertube Cross-Linking of Carbon Nanotubes at Room Temperature. Nano Letters 16 (10), 6541–6547 (2016) DOI: 10.1021/acs.nanolett.6b03184
Contact Information:
Prof.CHEN Liwei, i-Lab, Suzhou Institute of Nano-Tech and Nano-Bionics, Email: lwchen2008@sinano.ac.cn
downloadFile
