Rechargeable aqueous zinc metal batteries (AZMBs), as a high-safe and low-cost electrochemical energy storage technology, have recently gained increasing attention owing to high capacity (820 mAh g-1) and moderate redox potential (-0.76 V vs. SHE) of zinc anode. However, the hydrogen evolution reaction (HER) and Zn corrosion as well as rampant zinc dendrite growth leads to unsatisfactory plating/stripping reversibility and low Coulombic efficiency (CE). The performance of Zn anode is further degenerated especially under high current density or low-temperature surroundings, ultimately bringing about short-circuit and battery failure. In essence, the above problems result from the imperfect Zn ion behaviors at the electrode/electrolyte interface, which suffers from the large desolvation energy barrier of [Zn(H2O)6]2+ cluster and thedepressive Zn2+migration kinetics under low temperature. Therefore, to realize eventual practical application of AZMBs, the electrode/electrolyte interface must be rationally designed to strengthen the desolvation capability and ion diffusion kinetics.
Prof. LIN Hongzhen’s research group in Suzhou Institute of Nano-tech & Nano-bionics (SINANO),Chinese Academy of Sciences (CAS), is collaborated with Dr. Jian Wang in Germany Helmholtz Institute Ulm and has engaged in developing robust zinc metal anodes for advanced batteries. They have co-published an article entitled “Superfast Zincophilic Ion Conductor Enables Rapid Interfacial Desolvation Kinetics for Low-Temperature Zinc Metal Batteries” in Advanced Science to discuss the importance of lowing desolvation energy barrier of [Zn(H2O)6]2+.
In this work, LIN’s group member devised the superfast ionic conductor for achieving fast Zn2+ desolvation and efficient ion transport, which accelerates the Zn2+ transport kinetics and guides uniform Zn deposition. As examined comprehensively by in-situ/ex-situ characterizations including theoretical simulation and electrochemical measurements, the layered zinc silicate nanosheets (LZS) with abundant zincophilic sites and transport channels exhibits superior capabilities in accelerating desolvation of hydrated [Zn(H2O)6]2+ and promoting rapid diffusion of free Zn2+.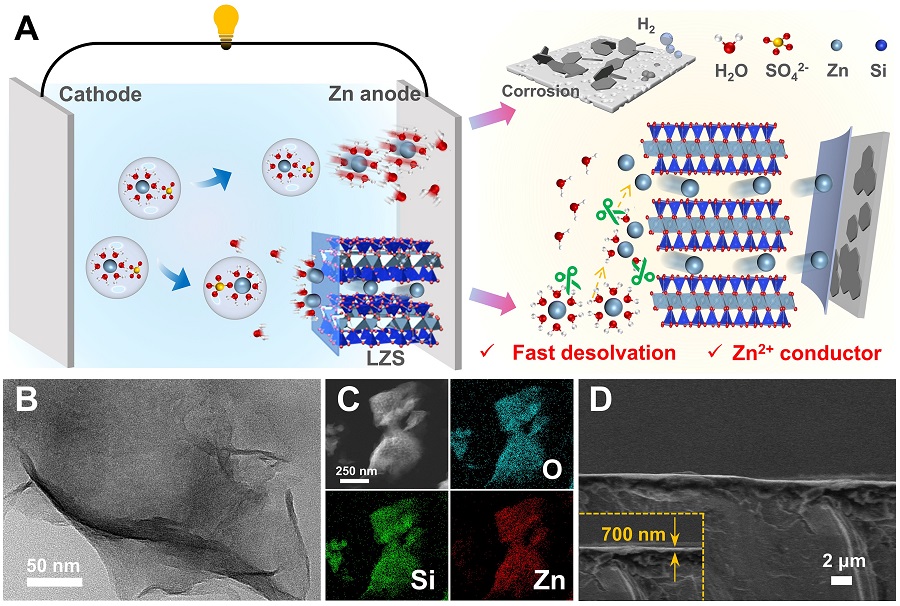
Figure 1. Schematic illustration of anode/electrolyte interface in accelerating desolvation and promoting Zn2+ diffusion kinetics.(Image by SINANO)
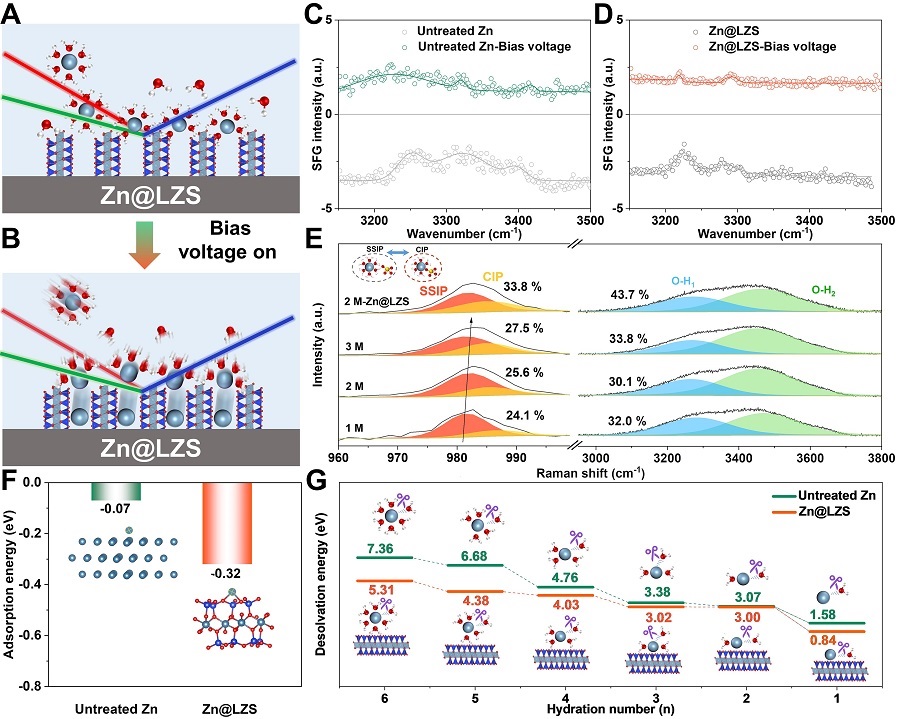
Figure 2. In/ex situ spectroelectrochemical characterizations and DFT calculations showcasing accelerated interfical Zn2+ dissociation.(Image by SINANO)
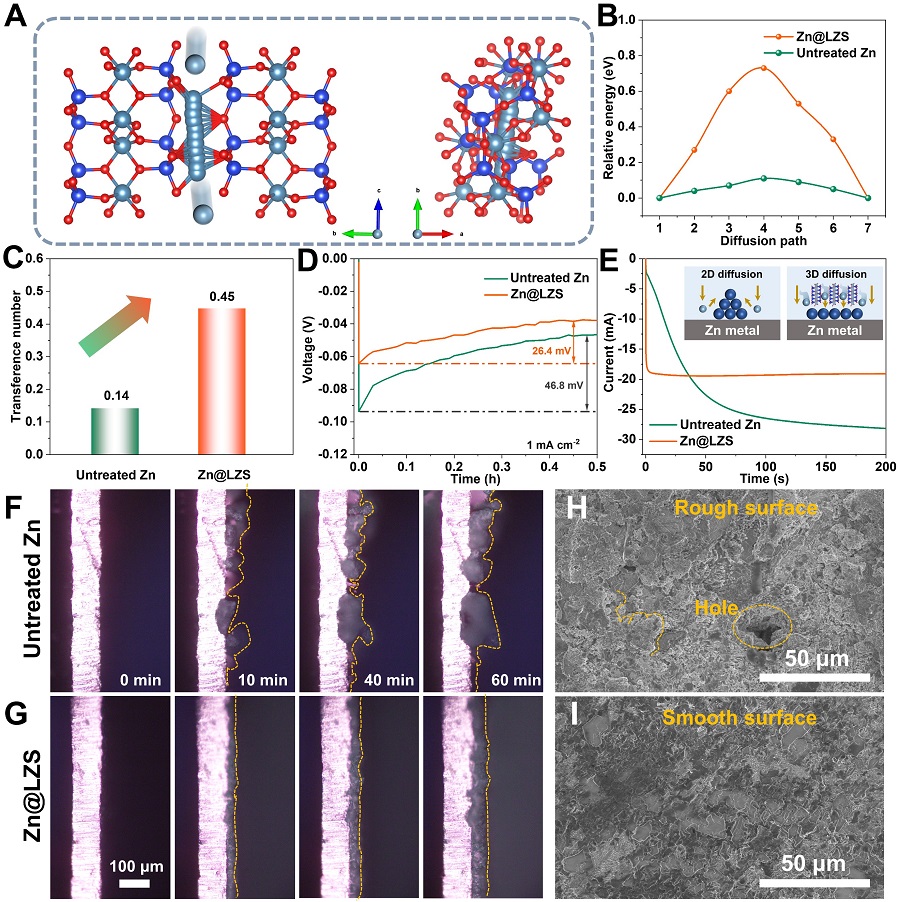
Figure 3. Spectroelectrochemical characterizations and In situ optical microscopy showcasing uniform Zn2+ deposition.(Image by SINANO)
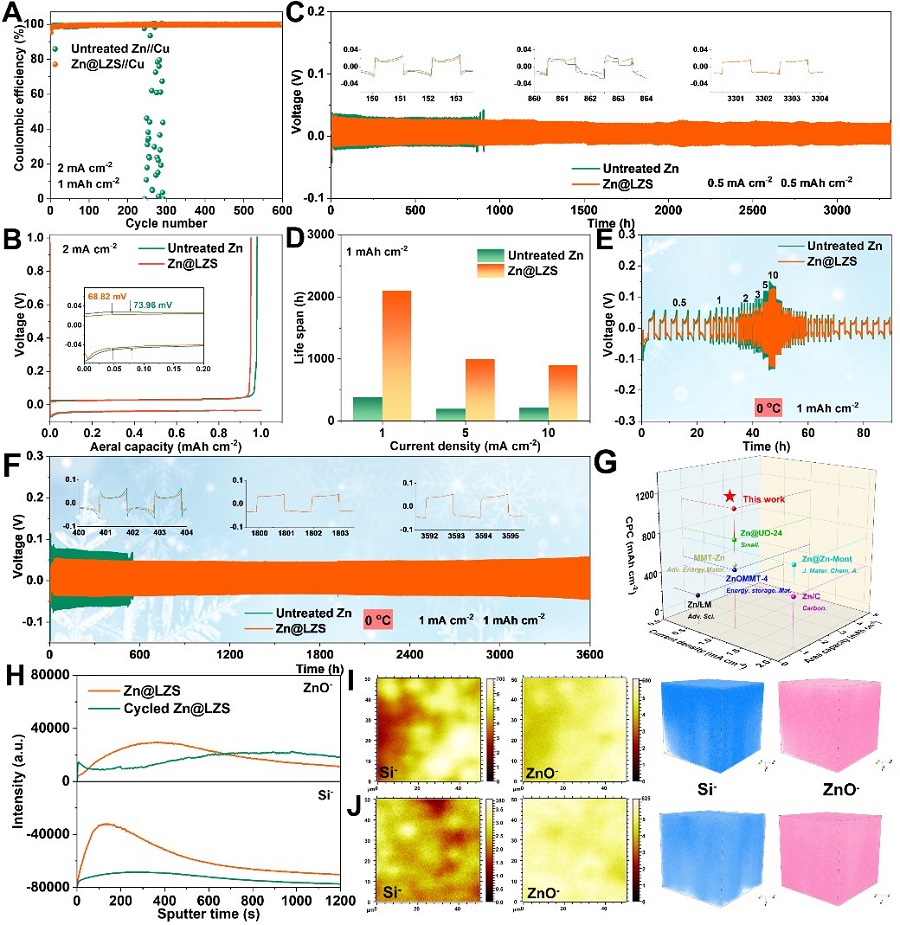
Figure 4. Dendrite-free Zn plating performance with Zn@LZS.(Image by SINANO)
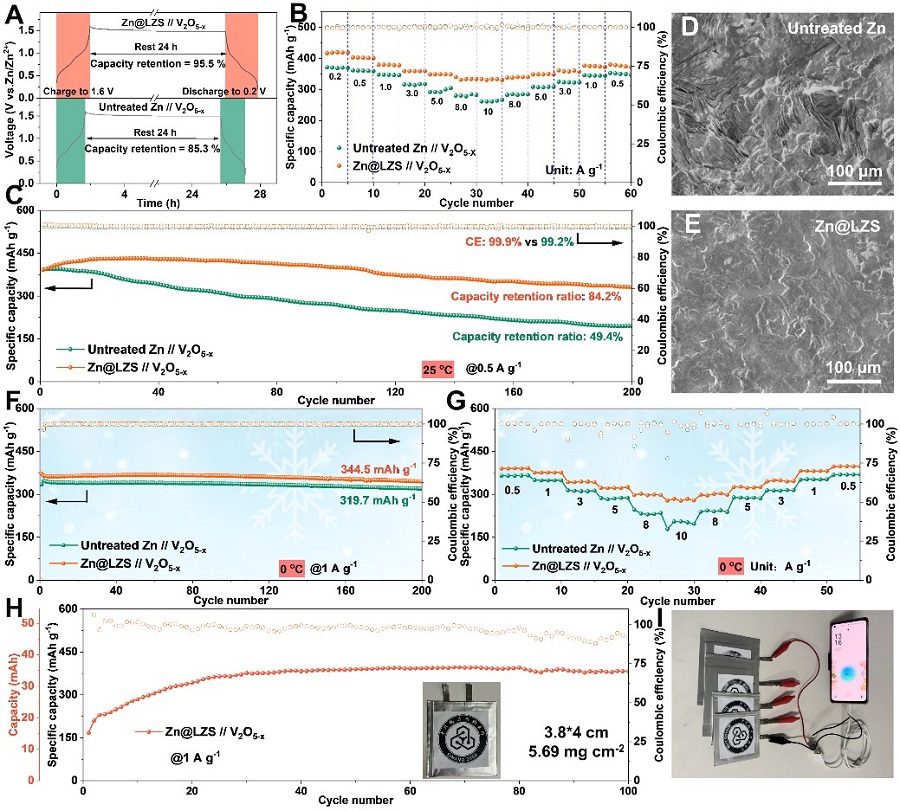
Figure 5. Electrochemical performance of full batteries based on the Zn@LZS anode. (Image by SINANO)
Benefiting from the rapid desolvation and the enhanced Zn2+ transport kinetics, the Zn nucleation and growth behaviors are significantly optimized. Dense and flat surface without Zn dendrite is achieved on Zn@LZS anode during the plating and stripping process. The LZS modified Zn anode showcases an ultralong cycling stability (> 3600 h) and distinguished rate performance at 0 oC. Extremely surprised, the assembled large-sized pouch cell renders a high capacity of 367 mAh g-1 after 100 cycles, which successfully powers the phone, pointing out the possibility for commericialization.
Publication Link:
X. Cheng, Y. Zuo, Y. Zhang,* X. Zhao, L. Jia, J. Zhang, X. Li, Z. Wu, J. Wang,* H. Lin,*Superfast Zincophilic Ion Conductor Enables Rapid Interfacial Desolvation Kinetics for Low-Temperature Zinc Metal Batteries. Adv. Sci. 2024, 2401629.
http://doi.org/10.1002/advs.202401629





