Recently, Prof. LIN Hongzhen's research group from Suzhou Institute of Nano-Tech and Nano-Bionics (SINANO) , Chinese Academy of Sciences(CAS), cooperated with Prof. WANG Shuangyin (Hunan University) and Dr. WANG Jian (Helmholtz Institute Ulm) had initially reported the Self-Tandem Catalysis for Ultrahigh-Performance Mg-S Battery. The research results were published online on the journal Energy & Environmental Science with the title " Self-Tandem Catalysis of Fast Mg2+ Desolvation and Sulfur Conversions for Ultrahigh-Performance Mg-S Battery via Serially-Assembled Atomic Reactors" (DOI: 10.1039/D3EE04028C).
Rechargeable magnesium-sulfur (Mg-S) batteries are famous for their high volumetric energy density and much improved safety without possible dendrite formation. However, the performances of Mg-S batteries are severely hindered by sluggish and dissatisfactory electrochemical kinetics of interfacial Mg2+ desolvation and successive sulfur species conversions. Here's what we have to note, an essential problem in secondary batteries based on conversion-type electrodes is that: for the redox conversion reactions to take place smoothly and efficiently, a prerequisite fundamental step, often been overlooked in the physical models described in the current literature, is the rapid desolvation of the charge carriers. The desolvation process has typical features of a chemical reaction (or a combination of a series of chemical steps) with substantially high energy barriers, which can be comparable or even larger than that of the subsequent conversion step. In particular systems of Mg-S in our contribution, this problem is even severe.
In this work, LIN’s group and others pioneered a self-tandem catalysis for achieving fast Mg2+ desolvation and sulfur conversions, and designed the serially-assembled train-like atomic reactors of zinc atomic catalysts employed onto long-conductive nitrogen-doped nanocarbons (STAR@LCNC), serving as electrochemical kinetic promotors. Benefited from these, the STAR@LCNC provides the capability in propelling dissociation kinetics of bridged MgCl(THF)x+ to form more Mg2+, and then guarantees high-speed electron exchange and highly active catalytic capability in catalyzing sulfur species at the interface, as thoroughly demonstrated from experimental and in-situ spectroscopical characterizations i.e. sum frequency generation spectroscopy to theoretical calculations. The so-fabricated sulfur cathode with STAR@LCNC delivers a long lifespan (400 cycles at 0.5 C) and the highest rate performance (612 mA h g-1 at 2 C) in the Mg-S battery systems reported so far. Also, the high sulfur loading cathode (4 mg cm-2) is capable of stabilizing 2.92 mA h cm-2 for 50 cycles, showing a bright future of tandem catalysis for practical batteries. This work opens up new routes to design and fabricate highly active catalysts with favorable interface environments to develop faster, longer-lasting, higher-energy density Mg-S batteries.
GUAN Qinghua, WANG Jian and ZHUANG Quan are co-first authors of this paper. Prof. LIN Hongzhen, Prof. WANG Shuangyin and Dr. WANG Jian are the co-corresponding authors. This research was financially supported by the National Key Research and Development Program, the National Natural Science Foundation of China, the Natural Science Foundation of Jiangsu Province, Innovative and Entrepreneurial Doctor in Jiangsu Province, Shanxi Natural Science Basic Research Plan as well as the technical support from Nano-X, Suzhou Institute of Nano-tech and Nano-bionics, Chinese Academy of Sciences. Dr. J. Wang thanks the funding provided by the Alexander von Humboldt Foundation.
Original link for the paper:
https://pubs.rsc.org/en/content/articlelanding/2024/ee/d3ee04028c
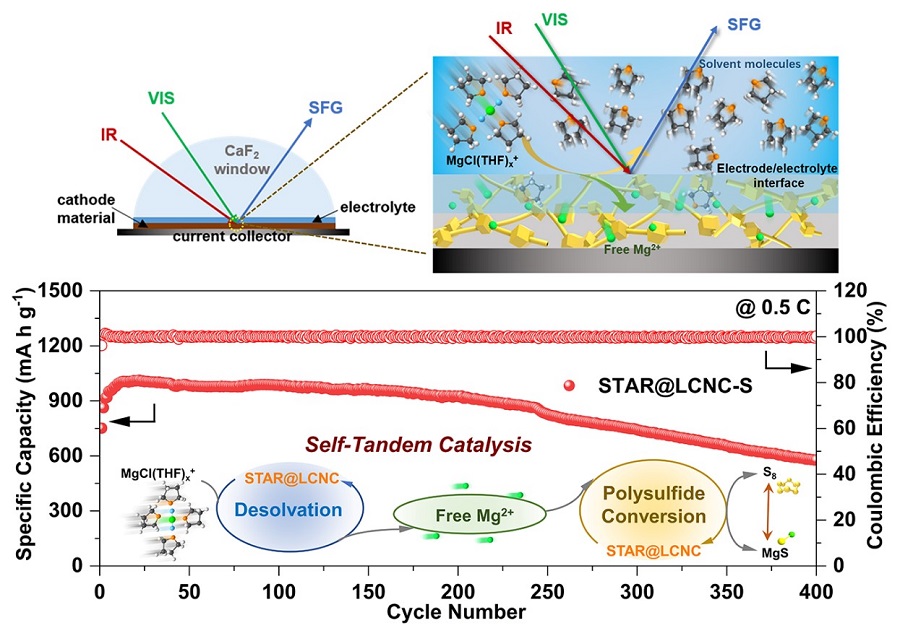
Fig 1. Schematic illustration of the in-situ SFG probing the electrolyte/catalyst interface.(Image by SINANO)
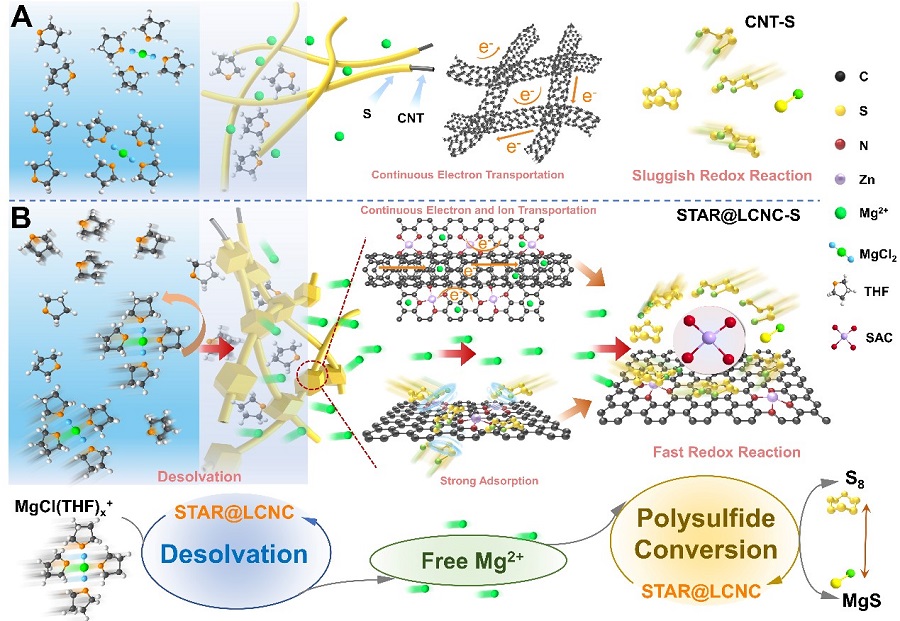
Fig 2. Schematic illustrations of atomic reactors in triphasic interfaces.(Image by SINANO)
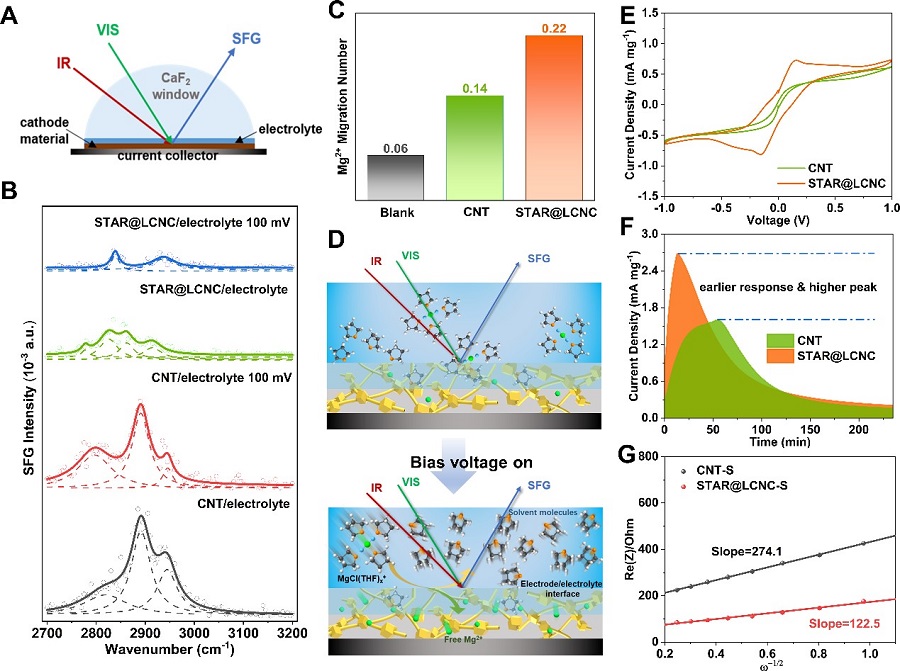
Fig 3. Experimental of interfacial desolvation and sulfur species kinetics catalyzed by STAR@LCNC.(Image by SINANO)
Contact information: Prof. LIN Hongzhen, Suzhou Institute of Nano-Tech and Nano-Bionics, Chinese Academy of Sciences
Email: hzlin2010@sinano.ac.cn
Open Access link: https://doi.org/10.1039/D3EE04028C
downloadFile
