Heteroaromatic containing chalcogen (oxygen, sulfur, selenium et al.) in fused aromatic ring systems have been actively investigated because of their remarkable optic and electronic properties for organic electronics. By introduction of oxygen atoms to the aromatic ring system, peri-Xanthenoxanthene (PXX) have received increasing attention by scientific research and application because their unique structures and excellent environmental stability. Furthermore, the molecular has multiple substitution positions, which can be modified with different functional groups to further regulate the optoelectronic properties and crystal packing modes.
Recently, Lv et al. from Printable Electronics Research Centre, SINANO, CAS, has developed a simple and efficient method for the preparation of novel type of PXX derivatives with alkyl in different substituent positions, the solubility was significantly improved compared to PXX. Moreover, these polycyclic heteroaromatic compounds exhibited different electronic and crystal structures according to UV−Vis spectra, electrochemical measurements, and X-ray structural analyses. They all show promising hole-transporting ability in the crystalline state. Furthermore, the substitution position can influence the solid state packing mode and charge transporting properties. Solution-processed organic thin film transistors (OTFTs) with 1,7-DOPXX have shown high apparent mobility over 0.5 cm2/V s and a high on/off ratio simultaneously. Related result has been published in Organic Letters (DOI: 10.1021/ol400790d)
This work was supported by NSFC (91123034, 21104091), the Knowledge Innovation Programme of CAS (KJCX2-EW-M02).
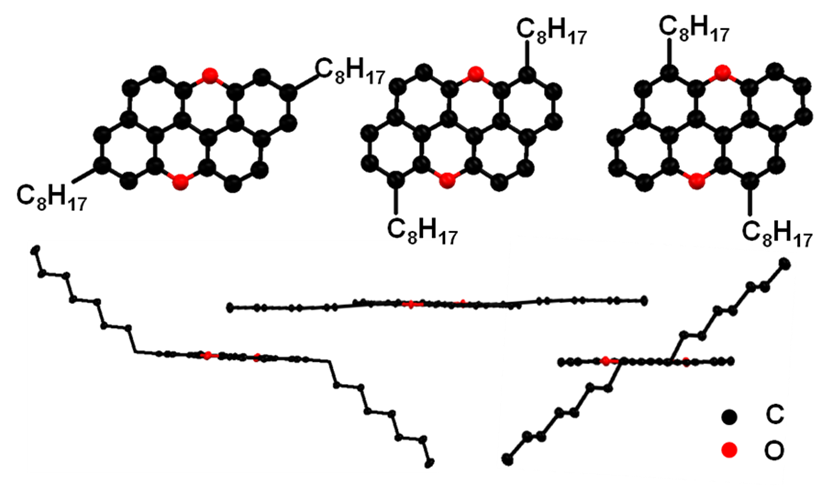
Figure 1. Molecule Structures of M1 (a), M2 (b) and M3 (c).(Image by SINANO)
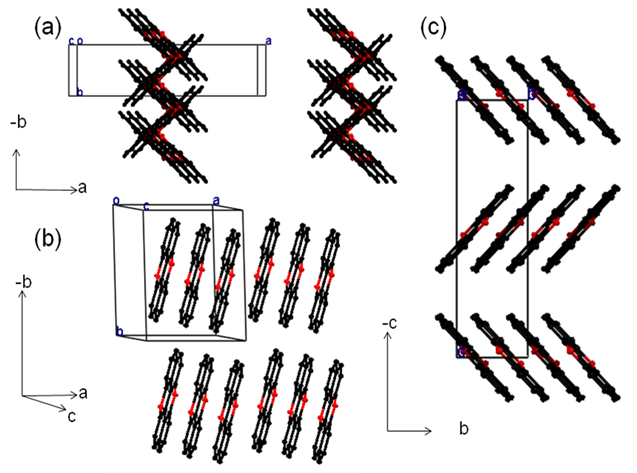
Figure 2. Crystal structures of M1 (a), M2 (b) and M3 (c). (Image by SINANO)
Figure 3. FET characteristics of M2: (a) transfer plots and (b) output plots. (For M1 and M3, see Supporting Information). (Image by SINANO)

