Rapidly growing demands for electrochemical energy storages are driving scientists around the world to develop next-generation high energy density lithium battery. Lithium sulfur (Li-S) battery, based on reversible redox reactions between sulfur cathode and lithium metal anode, promises a theoretical energy density as high as 2600 Wh/Kg, therefore, has great potential in applications such as EVs, HEVs and 3C electronic products.
One of the technological bottlenecks in Li-S batteries is the insulating nature of elementary sulfur and the discharging products Li2S2 and Li2S. The slow charge transfer process in the cathode structure thus imposes restrictions on the degree of sulfur utilization, initial capacity and the rate performance.
Recently, Dr. Liwei Chen’s research group in the Suzhou Institute of Nano-Tech and Nano-Bionics(SINANO), Chinese Academy of Sciences, has made progresses in understanding and improving the cathode charge transfer process in Li-S batteries.
The investigation focused on the nano-size effect in S cathodes. As the first step, S nanoparticles with diameters below 50 nm were fabricated via a membrane-assisted precipitation method. With conductive PEDOT coating, these S nanoparticles show high specific capacity and improved cycle stability (figure 1). The study indicated that nanosized sulfur required shorter charge diffusion distance and thus showed higher capacity (Scientific Reports.2013,3,1910). Further understanding of the nano-size effect needs better size-controlled S materials, which were obtained using the sulfur-amine chemistry developed in the Chen group (Chemical Communications,2014,50,1202). A series of mono-dispersed S nanoparticles with diameters ranging from 5 nm to 150 nm were employed to investigate the size-dependent charge transfer process and the influence on device performance. Experimental results revealed much improved specific capacity, rate performance and cycle stability upon the reduction of S nanoparticle size. Importantly, the 5 nm S particles showed an initial capacity of 1670 mAh/g, which is the theoretical limit of sulfur. This work demonstrates that the high theoretical performance can indeed be realized (Nano Letters. 2015, 15, 798). The understanding of S cathode charge transfer will help future cathodic design in the Li-S technology.
Based on the basic scientific understanding, the Chen group is also committed to develop practical Li-S pouch battery, aiming at solving engineering challenges towards the commercialization of Li-S batteries (figure 3). State-of-the-art Li-S pouch battery has reached the energy density > 400 Wh/kg, or lifetime over 50 cycles, respectively.
This series of research are funded by the National Natural Science Foundation of China, the Strategic Priority Research Program of Chinese Academy of Sciences, and the Suzhou Institute of Nanotech and Nanobionics,Chinese Academy of Sciences.
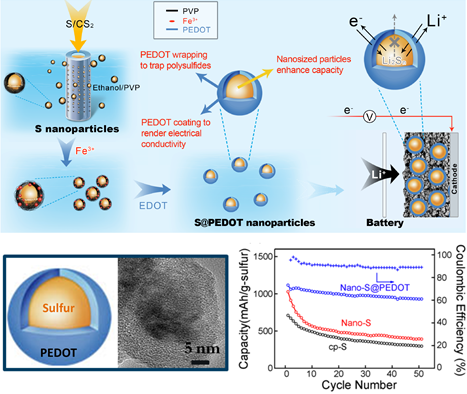
Figure 1. Schematic illustration of PEDOT coated S nanoparticles and their electrochemical performance (Scientific Reports.2013,3,1910).(Image by SINANO)

Figure 2. Electrochemical performance of 5nm S particles. (Nano Letters. 2015, 15, 798) (Image by SINANO)

Figure 3. Photograph of prototype Li-S pouch batteries. (Image by SINANO)

