The rapid development of society and industry is Increasingly demanding for higher electrochemical energy storage capabilities. The development of high-capacity cathode materials is critical for applications such as mobile devices and electric vehicles. The emerging rechargeable lithium sulfide (Li2S) batteries hold great potential for high-performance energy storage systems because they have a high theoretical specific energy, low cost, and are eco-friendly.
Li2S is a promising prelithiated cathode material with a high theoretical capacity of 1166 mA·h·g-1. Li2S cathode shrinks as it delithiates initially, producing voids that could act as buffer space for volume changes during the subsequent lithiation/delithiation cycling, and hence protecting the electrode structure from mechanical damage. More importantly, Li2S can be matched with lithium metal-free anodes (such as silicon and tin), thus eliminating serious safety issues associated with the formation of “dendrites.” Despite of these merits, the performance of Li2S-based cathode significantly lags behind its sulfur counterpart. A key issue for the Li2S material seems to be in its inefficient activation and redeposition process, these factors prevent the practical application of Li2S batteries.
Recently, ZHANG Yuegang’s group at Chinese Academy of Sciences used their newly-developed in-situ SEM and TEM chips, and realized real-time observation of the charging process of lithium sulfide electrodes. Based on the understanding of the material’s charging mechanism, they synthesized a composite Li2S cathode using a highly nitridated graphene (HNG) and developed a new charging scheme by controlling the charge capacity and voltage, which significantly improved the capacity utilization rate and cycle life. The results were recently published on Advanced Energy Material.
To improving the capacity utilization rate and cycle life of Li/S batteries, much effort has been placed on encapsulating sulfur into conductive matrixes with porous structure and/or high surface to volume ratio, such as carbon nanotubes, porous carbon, graphene, and carbon fibers. In their previous research, ZHANG’s group has found that N-doped graphene can effectively alleviate the dissolution of polysulfide and improve the redeposition of lithium polysulfides (Nano Letters, 2014, 14, 4821-4827). To better improve the capacity utilization rate and cycle life of Li2S cells, this group studied the mechanism of polysulfide dissolution and redeposition, and proposed a new charging scheme: an initial charge to 3.8 V versus Li/Li + to activate Li2S, followed by cycling in a limited voltage window from 1.7 to 2.4 V and with a controlled charge capacity. This scheme can alleviate formation of soluble long-chain polysulfides. The insoluble Li2S residual during the modulated charge process can be used as seeds for a uniform redeposition of active Li2S materials and adsorption of soluble intermediate polysulfides.
On the materials synthesis side, wrapping graphene oxide with glucose before nitridation resulted in a final product with more curvature and wrinkles, which provided more anchor points for absorption of intermediate polysulfides. Using ammonia and a high temperature nitridation treatment method increased the N-doping amount up to 12.2% in the final product. The highly nitridated graphene can not only provides higher conductivity, but also alleviate dissolution of polysulfides in the electrolyte and help to redesposit Li2S more uniformly on the graphene scaffolds via the high density nitrogen functional groups. The cell using the HNG-Li2S composite cathode delivered a specific capacity of ≈318 mA·h·g -1 (≈457 mA·h·g -1 of S) after 2000 cycles at a 1C discharge rate. More impressively, when discharged at 2 C, the cell could still deliver a specific capacity of ≈256 mA·h·g -1 (≈368 mA·h·g -1 of S) even after 3000 cycles, which is the best cycle-life reported so far for Li2S batteries to the best of our knowledge.
This work utilized the newly-developed in-situ SEM chip and in-situ TEM chip to realize the real-time observation of the lithium sulfide electrode charging process. The new modulated voltage-capacity charging scheme and the designed HNG-Li2S composite cathode could open a promising avenue for the practical implementation of high-energy density Li2S-C/Li cells.
This work was supported by the National Natural Science Foundation of China and The Recruitment Program for Innovative Talents of China.
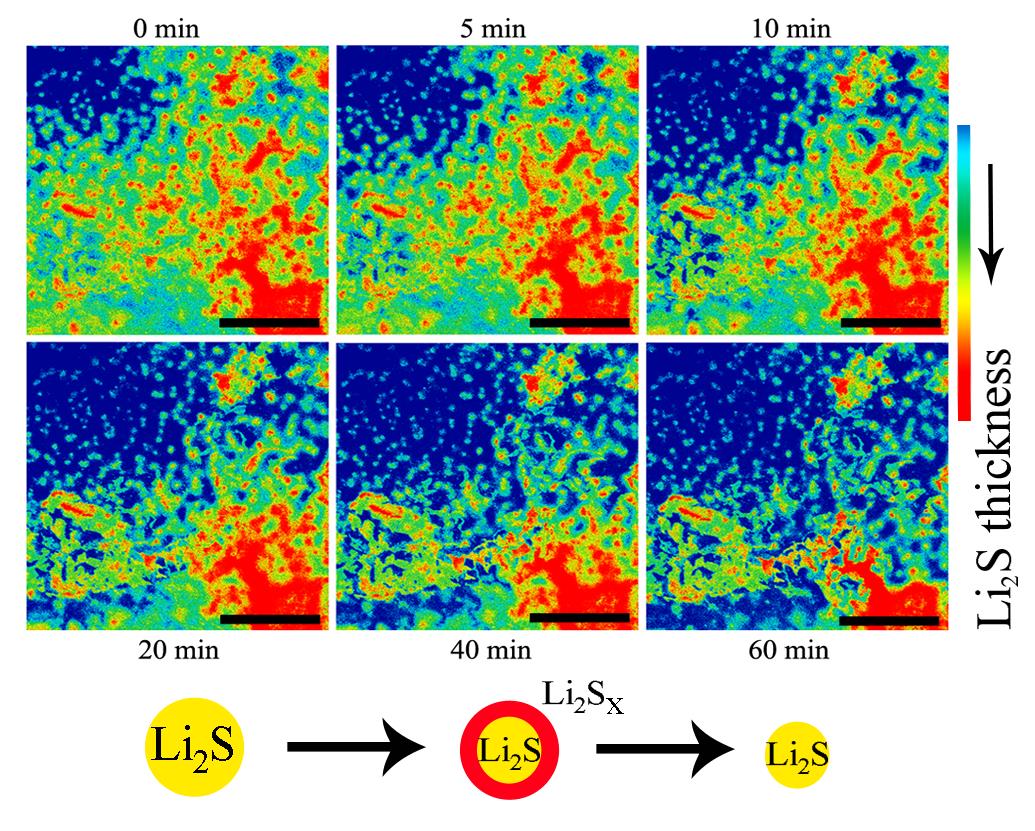
Figure 1: Time-lapse SEM images of the activation process of Li2S on a single-layered graphene electrode in a standard LiTFSI-DOL/DME electrolyte.(Image by ZHANG Yuegang’s group)
Contact information:Prof. ZHANG Yuegang
Suzhou Institute of Nano Tech and Nano Bionics ,Chinese Academy of Science
Suzhou,Jiangsu 215125,China.
E-mail:ygzhang2012@sinano.ac.cn
http://www.materialsviewschina.com/2015/10/lithium-sulfide-battery-by-in-situ-electron-microscopy-and-cycle-stability-control-made-significant-progress/

