Isolation and characterization of circulating tumor cells (CTCs), which have been considered as a biomarker of cancer metastasis, is of great importance to provide critical information for diagnosis of cancer and personalized therapy. Numbers of techniques for CTC detection have been developed during the past decades, including cell size-based isolation, immunomagnetic beads and microfluidic chips, which can successfully detect the presence of CTCs in metastatic cancer patients. However, it is still challenging to achieve viable and pure CTC samples, which is necessary for the subsequent molecular characterization and functional analysis, conceived to be of great significance for revealing the mechanism of tumor biology, cancer metastasis and drug sensitivity. Recently, PEI Renjun’s team from SINANO, CAS reported their research progress in specific capture and non-destructive isolation of CTCs based on nano-structured substrates as well as aptamers.
With the development of nanotechnology, it is well-known that cells can sense and interact with the nanotopography of materials. The importance of nanomaterials has been constantly tested with the exploration of the interaction between cells and the nanotopography of materials. In the past decade, nanostructured materials have been incorporated with CTC detecting devices, and the design of nanomaterials becomes the key to solve these challenges now. Pei’s team prepared a uniform multiscale TiO2 nanorod array to provide a “multi-scale interacting platform” for cell capture. The TiO2 nanorods are densely packed on F-doped SnO2 (FTO) substrates with diameter of 160-300 nm, providing a 3D interface for cell contact, and the nanorods are composed of nanoparticles of 30-50 nm, which might further enhance the topographic interaction between nanoscale structures on cell surface and TiO2 nanorod array. The capture yield of artificial blood samples on the BSA-aptamer TiO2 nanorod substrates is up to 85%-95%, revealing the potential application of the TiO2 nanorods on efficient and sensitive capture of rare circulating tumor cells. (ACS APPLIED MATERIALS & INTERFACES, 2016, 8, 12638-12643)
Nanostructures can greatly improve the efficiency of CTC capture efficiency, but at the same time a large number of non-specific adsorption of blood cells in the blood also increases, resulting in decreased purity CTC capture. To solve this problem, the research team fabricated a chitosan nanoparticle surface by electrospray to provide a cellular compatible interface. The “soft” substrate, further modified by polyethylene glycol (PEG) as an antifouling molecule and DNA aptamer as a specific capture molecule, has a hydrophilic nature and is capable of specific capture of viable rare CTCs from artificial white blood cell (WBC) samples. Furthermore, inspired by “survival of the fittest,” they introduced a novel strategy in this study for further purification and proliferation of the rare number target cells through the following optimized in situ culture of captured cells, as described in Figure 1 .
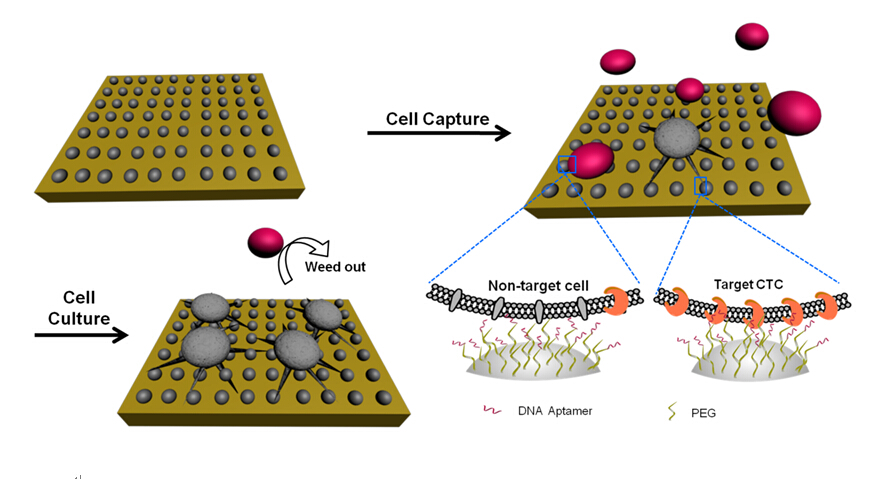
Figure 1. Schematic of the chitosan nanoparticle substrate for rare number CTC isolation from nontarget cells followed by the optimized in situ culture of captured cells. The in situ culture realized the proliferation of rare number CTCs and simultaneously weeded out captured nontarget cells (e.g., WBCs) to obtain pure target CTCs for further analysis.(Image by SINANO)
To realize the recovery of viable CTCs without any foreign agents, it is crucial to design appropriate interfacial chemistry according to a certain nanostructure on account of diverse interaction mechanisms between cells and nanostructures. A nanostrucure of porous network based on chitosan nanofibers was fabricated by electrospinning, to mimic the function of extracellular matrices, and then the pCBMA brushes integrating onto nanofiber interface provided the effect of interfacial properties to control non-specific cell adhesion and the multivalent immobilization of aptamers to induce high efficient and specific CTC capture. Furthermore, a complementary sequence (CS) was used to efficiently hybridize with the aptamer to achieve nondestructive release of the captured target cells, assisted by the flexible space provided by pCBMA brushes, as shown in in Figure 2.
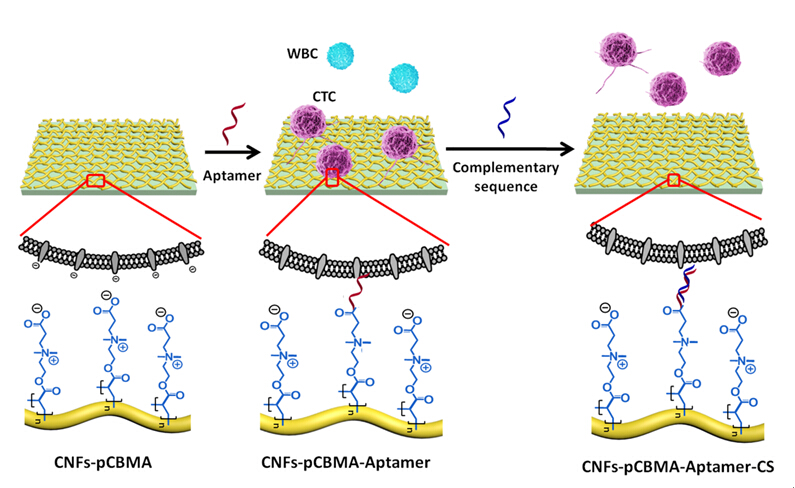
Figure 2. Schematic illustration of the design of bio-interface for specific capture and release of cancer cells.(Image by SINANO)
Contact information: Prof. PEI Renjun
Suzhou Institute of Nano-Tech and Nano-Bionics, Chinese Academy of Science
Suzhou, Jiangsu 215123, China.
E-mail: rjpei2011@sinano.ac.cn

