Gas-generating catalysis is important to many energy-related research fields, such as photocatalytic water splitting, water electrolysis, dehydrogenation, the electro-oxidation of small organic molecules, etc. The technique of single-nanoparticle catalysis is an effective way to search for highly active nanocatalysts and elucidate the reaction mechanism. However, gas-generating catalysis remains difficult to investigate at the single-nanoparticle level because product gases, such as H2 and O2, are difficult to detect on an individual nanoparticle, particularly when the gases are dissolved in solution.
Recently, ZHOU Xiaochun's group in the Suzhou Institute of Nano-Tech and Nano-Bionics (SINANO), Chinese Academy of Sciences,has found that nanobubbles can be used to study the gas-generating catalysis. Related results were published in Journal of the American Chemical Society under the title “Nanobubbles: An Effective Way to Study Gas-generating Catalysis on a Single Nanoparticle”.

Track gas-generating catalysis on a single nanoparticle by monitoring the nanobubble via DFM. (a) TEM image of Pd-Ag nanoplate catalysts. (b) Experimental scheme using DFM and a microfluidic reactor to image the nanobubble containing the gas from the dehydrogenation of FA on a single Pd-Ag nanoplate. (c) Schematic of the light scattering of a nanobubble on a single Pd-Ag nanoplate catalyst. (d) Wide-field DFM image of individual Pd-Ag nanoplates undergoing FA dehydrogenation and dodecyl sulfate (SDS). The scale bars in the inserts are 100 nm. (e) The scattering intensity versus time trajectory for the evolution of nanobubbles on a single Pd-Ag nanoplate. (Image by SINANO)
Through a series of control experiments, this research successfully demonstrates that the gas-generating reaction on a single Pd-Ag nanoplate catalyst can be detected by monitoring the nanobubbles by DFM. And they also found that the nanobubble evolution process includes nucleation time and lifetime.
In order to obtain the relationship between nucleation rate of nanobubbles and activity of a single nanocatalyst, the researchers studied the temporal nucleation rate of the nanobubbles on individual Pd-Ag nanoplates in the presence of 1.67 M of FA and the nucleation rate of a nanobubble with time for different FA concentrations. The results show that the nucleation rate of nanobubbles is proportional to the catalytic activity of a single nanocatalyst. Authors also built a mathematical model to quantitatively describe the relationship between the catalytic activity and the nucleation rate, which shows that an onset reaction rate (ronset) exists for the generation of nanobubbles on a single Pd-Ag nanoplate. And a remarkable number of nanobubbles will be generated on a single nanoplate only when the reaction rate of a single Pd-Ag nanoplate is higher than the onset rate. This model provides a method to determine the activity of a single nanocatalyst through the measurement of the nucleation rate of the nanobubbles generated on it.
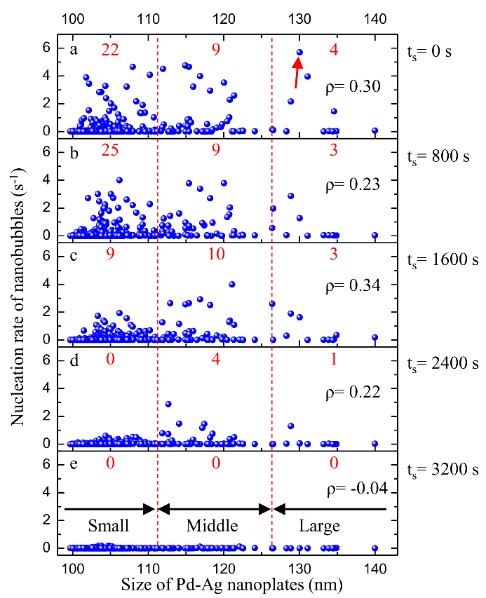
Time dependent nucleation rate of nanobubbles on different sizes of Pd-Ag nanoplates at 1.67 M of FA. (a) ts= 0 s. (b) ts= 800 s. (c) ts= 1600 s. (d) ts= 2400 s. (e) ts= 3200 s. The red numbers on the top of each plot are the counts of Pd-Ag nanoplates, whose nucleation rate is faster than 1 s-1. Pearson's correlation coefficient (ρ) for different time is shown in the top right of each plot. (Image by SINANO)
This research also reveals that the activity of single Pd-Ag nanoplates is related to the size and the Pd content of the Pd-Ag nanoplate. A Pd-Ag nanoplate with larger size usually has a higher activity, and the Pd content is the key factor for the activity of single Pd-Ag nanoplates with similar size.
This research studied the gas-generating catalysis by monitoring nanobubbles using DFM at a high spatial resolution (single nanoparticle) and at a high time resolution (50 ms). Because the gas-generating catalysis on single nanocatalyst is difficult to do, this research becomes significant in providing an effective way to find the highly active nanocatalyst and study the mechanism of nanocatalyst for gas-generating reaction at the single-nanoparticle level, enabling further investigation in many other energy-related fields, such as water splitting and the electro-oxidation of small organic molecules.
Links: Website of ZHOU Xiaochun's group: http://www.mscatalysis.net/
Literature: Nanobubbles: An Effective Way to Study Gas-generating Catalysis on a Single Nanoparticle. 2017, 139(40), 14277-14284.

