Electrochemical water splitting is regarded as one of the most efficient ways to produce molecular hydrogen. The oxygen evolution reaction is a very important half-reaction involved in water splitting but this process always suffers from sluggish kinetics due to the multiple four electron steps, so it requires catalysts to accelerate the process. Precious metal based materials, such as ruthenium dioxide and iridium dioxide, are the state-of-the-art OER electrocatalysts, but their high cost and low reservation limit their wide applications. Therefore, it is desired to develop high efficient OER catalysts with low cost and high abundance.
Nickel-based material is a very popular non-precious metal based catalyst and has drawn intense attention, for example nickel oxides, hydroxides, phosphides, nitrides, chalcogenides and their derivatives. Recently, some works suggest that the high-valent Ni in nickel-oxide-based materials can greatly enhance their OER performances since the high valent Ni is closer to the active oxidation state in OER region. So, further understanding of the relationship between catalytic activity and chemical valence of Ni component in nickel-based compounds is very important, but it remains challenging, because it is hard to achieve the controllable synthesis of catalysts with tunable valence but uniform shape and size.
Recently, Prof. WANG Qiangbin’s group from Suzhou Institute of Nano-Tech and Nano-Bionics (SINANO), Chinese Academy of Sciences (CAS) used a very simple method to synthesize a series of nickel sulfides hybridized with N and S co-doped carbon nanoparticles (NixSy-NSCs) with different chemical valences of Ni, Ni9S8-NSCs, Ni9S8-NiS1.03-NSCs and NiS1.03-NSCs, and then systematically investigated their electrocatalytic performances as oxygen evolution reaction electrocatalysts. The formation of NixSy-NSCs from Ni9S8-NSCs to Ni9S8-NiS1.03-NSCs and NiS1.03-NSCs is a sulfur enriched process, giving rise to the increase of high-valence Ni component, and resulting gradually enhanced OER electrocatalytic activities. In particular, the NiS1.03-NSCs show an exceptional low overpotential of ~270 mV vs. RHE at a current density of 10 mA cm-2 and a small Tafel slope of 68.9 mV/dec with mass loading of 0.25 mg cm-2 in 1 M KOH and remain their catalytic activities for at least 10 h.
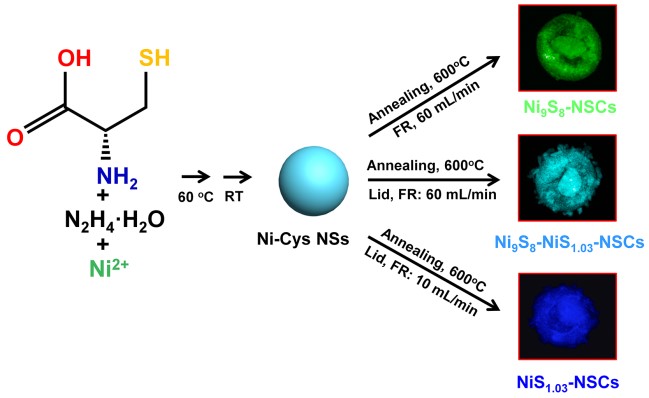
Figure 1. The synthetic process of NixSy-NSCs(Image by SINANO)

Figure 2. OER performances of these as-prepared NixSy-NSCs(Image by SINANO)
We expect that our new understanding of the chemical valence-dependent electrocatalytic activity will pave the way for rational design of high-efficient electrocatalysts and the novel NixSy-NSC NPs with excellent OER electrocatalytic performance and superior stability will be promising alternatives to the precious-metal-based catalysts to OER, and meanwhile this unique synthetic strategy to fabricate NixSy-NSC hybrids could be expanded to synthesize many other functional materials.
This work was financially supported by the National Natural Science Foundation of China (Grant Nos. 81401464, 21425103, and 21501192) and the National Key Research and Development Program (2016YFA0101503, 2017YFA0205503).
Contact information:
Prof. WANG Qiangbin, Suzhou Institute of Nano-Tech and Nano-Bionics, Chinese Academy of Sciences
Email: qbwang2008@sinano.ac.cn
Reference: http://onlinelibrary.wiley.com/doi/10.1002/smll.201703273/full

