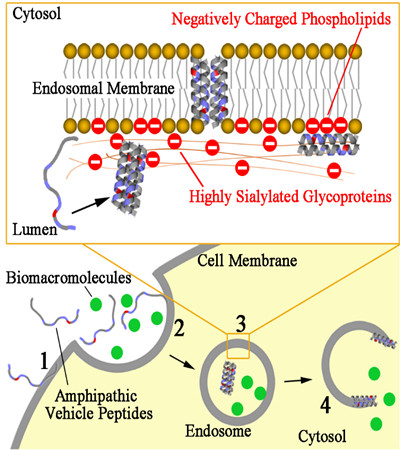
The cell plasma membrane and its derived endosomal membranes are both greasy barriers that refuse the entrance of water-soluble macromolecules, such as proteins and nucleic acids, into cells.
Breaking “doors” directly on plasma membrane could easily kill the cell, but opening inner “doors” on endosomal membrane, although safe, has been difficult to achieve.
A recent study published in Journal of Biological Chemistry reported a peptide that can safely open up endosomal membrane, so that cargos such as proteins can be delivered into the cytoplasm. Meanwhile, it leaves the plasma membrane intact.
The team led by Prof. Hao Fei from Suzhou Institute of Nano-tech and Nano-Bionics Chinese Academy of Sciences used a strategy of hydrophobicity-tuning on a set of artificial peptides and discovered an optimized peptide being particularly responsive to the acidic environment on the endosomal membrane.
These peptides are generally called “amphipathic peptide”, because they have both greasy (water repelling or hydrophobic) and polar (water attracting or hydrophilic) ends. The greasy end helps the peptide insert into membranes. The polar end helps the peptide remain water soluble and later form helical shapes to break holes in the membranes.
These peptides are also “cationic” or positively charged, as several amine-containing amino acids are used in their sequences. These cationic groups are attracted to the negatively charged acidic phospholipids and sialic acids (a type of sugar) often found in cell membrane but highly enriched in endosomal membranes.
Through the hydrophobicity-tuning strategy the team elegantly balanced the two key actions of the peptide: attaching itself to the cell membrane without causing cell death; and forming peptide assembled holes in the acidic endosomal membrane to allow cargo entry.
The resulting peptide LP6 has just necessary and sufficient hydrophobicity to endow its helical and aggregation ability under the acidic endosomal environment, and can dramatically promote cargo cell entry and cytosolic delivery of macromolecules such as dextran, protein toxin, and human immunoglobulin.
“From the reductionist point of view, the study used the simplest natural proteinogenic amino acids and the most essential structures of peptide, thus our finding may represent a structure-function relationship that is fundamental.” added Prof. Fei.
It is estimated that 75% of human proteome is intracellular and inaccessible to protein therapeutics such as monoclonal antibody. Moreover, many protein targets, including overexpressed oncogenic proteins, mutated tumor suppressors have been considered “undruggable” by small molecule inhibitors. Thus, the reported findings would be instructive for the rational design and modification of effective vehicle peptides for cytosolic delivery of macromolecular therapeutics for future biomedical application.