Figure 2. The schematic illustration of 4f-electron state modulation in realizing dendrite-free morphology.
In details, as displayed in Figure 3, the 4f electronic structure in CeO2 has the characteristics of tunability and orbital hybridization, inducing the disorder of the electronic states by introducing Schottky defects. Subsequently, the 4f electronic state is significantly recovered after interaction with Li atoms via charger transfer, which is a key factor for the uniform trapping of Li atoms during the initial nucleation process. In addition, the adsorption energy increases with the intensity of electronic manipulation together with the higher concentration of Schottky defect, which is more favorable for capturing Li atom and smooth plating.
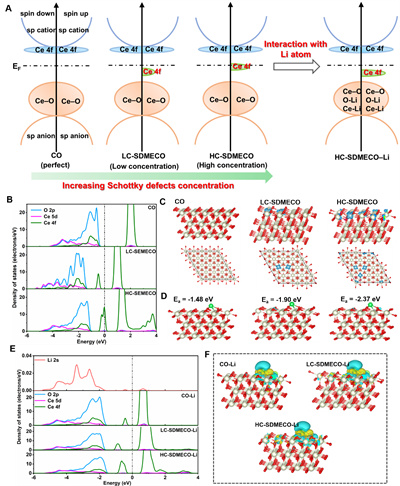
Figure 3. Theoretical simulation of the interaction between Schottky Defects in Cerium Oxide and lithium atoms.
Due to the rearrangement of the electronic structure of the 4f center of Ce, the generated numerous active sites enhance the catalytic activity of SDMECO@HINC and thus reduces the nucleation barrier of Li atoms down to 11 mV. During subsequent cycling, SDMECO@HINC enables faster diffusion of Li atoms and more uniform deposition with a stable overpotential of ~13 mV for 1200 h without dendrite formation. At the same time, the SDMECO@HINC catalytic layer could significantly improve the Coulombic efficiency up to about 98%. With the Scanning Electron Microscopy, the surface of Li metal after SDMECO@HINC modification is uniform and smooth without obvious volume change in contrast to the obvious crack in pristine Li electrode. The catalytic desolvation of the SDMECO@HINC modified layer is further verified by interface-sensitive in situ and frequency spectroscopy (SFG), which effectively promotes the generation of free Li+ from solvated Li+ for uniform Li deposition. As summarized in Figure 4, the principal mechanism of SDMECO@HINC is well depicted.
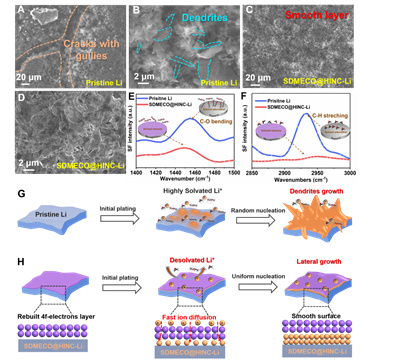
Figure 4. Modulation mechanism of SDMECO@HINC on the kinetics of lithium ions for smooth plating.
In their previous works on sulfur cathodes, Lin’s group has found that metal single-atom catalysts and defect catalysts can control the kinetic behavior of lithium ions (Energy Storage Mater 2019, 18, 246; Energy Storage Mater. 2020, 28, 375; ChemSusChem 2020, 13, 3404; Energy. Environ. Mater. 2021, DOI: 10.1002/eem2.12250; Adv. Energy Sus. Res. 2022, 2100187 ). Based on the gained knowledge, one would expect that metal single-atom catalysts on nanocarbons can help to provide abundant binding sites with proper lithiophilicility to modulate the diffusion kinetics of lithium, and to guide the uniform deposition of lithium without dendrite formation. However, apart from the wide preparation method of using nitrogen-doped nanocarbons to support SACs, there still lacks of simple and effective methods for anchoring and stabilizing high-concentration SACs.
For the first time, a strategy of using defect sites to anchor metal single atoms (SAC-in-Defect) is developed by Lin's group (Figure 5). Briefly, highly active metal single atoms are anchored in cationic defect compounds through hydrothermal and heating treatments, as revealed by STEM-HAADF and XAS. As the modulation promoter, lithium ion diffusion kinetic is propelled by the abundant metal single atom sites. In the later cycles, the SAC active sites can guide the uniform nucleation of Li with a lower energy barrier and promote the dendrite-free process on Li metal surfaces. Cycled at 1 mA cm-2, the prepared pouch battery with a sulfur loading of 5.4 mg cm-2 delivers an areal capacity of 3.78 mA cm-2, shedding light on the future for practical application (Nano Lett. 2021, 21, 3245).
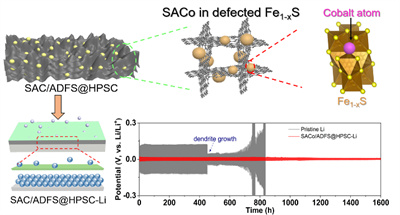
Figure 5. The schematic description of “SAC-in-Defect” catalyst in regulating lithium behaviors.
The above works successfully applied the state-of-the-art catalyst design strategies, such as defect engineering and atomic dispersion of active sites, in decreasing the energy barriers and propelling the lithium kinetics of ionic/atomic diffusion, desolvation, and transfer. The concept that these physical processes can be “catalyzed” in a similar way as a typical chemical reaction will greatly broaden the application range of electrocatalysts in secondary batteries.
Publication Link:
Tuning 4f-center Electron Structure by Schottky Defects for Catalyzing Li Diffusion to Achieve Long-term Dendrite-free Lithium Metal Battery, Jing Zhang, Rong He, Quan Zhuang, Xinjun Ma, Caiyin You, Qianqian Hao, Linge Li, Shuang Cheng, Li Lei, Bo Deng, Xifei Li, Hongzhen Lin, and Jian Wang, Adv. Sci. 2022, 20210468. DOI: 10.1002/advs.202202244
https://onlinelibrary.wiley.com/doi/10.1002/advs.202202244
Long-Life Dendrite-Free Lithium Metal Electrode Achieved by Constructing a Single Metal Atom Anchored in a Diffusion Modulator Layer, Jian Wang;Jing Zhang;Shuang Cheng;Jin Yang;Yonglan Xi;Xingang Hou;Qingbo Xiao;Hongzhen Lin, Nano Lett. 2021, 21, 3245-3253.
https://pubs.acs.org/doi/full/10.1021/acs.nanolett.1c00534
More details are available in the group website: www.hzlin.cn
Authors’ Profiles

Dr. Hongzhen Lin,a full professor in i-Lab of Suzhou Institute of Nano-tech and Nano-bionics, CAS, leads a research group in developing advanced electrochemical energy materials and batteries via novel catalytic strategies, and revealing the related interfacial mechanisms with in-situ sum frequency generation spectroscopy. He has published over 100 works on top journals such as Nat. Commun.、Sci. Adv.、JACS、Nano Lett.、Adv. Funct. Mater.、Angew.Chem. Int. Ed.、Adv. Sci.、Nano Energy、Energy Storage Mater.、J. Phys. Chem. Lett., as communicating or first author.
E-mail: hzlin2010@sinano.ac.cn

Dr. Jian Wang, granted by Humboldt Scholar fellowship, works in the Helmholtz Institute Ulm, KIT, in Germany. His research interest mainly focuses on interdisciplinary between electrocatalysis and second conversion-based batteries. He is devoted to exploring the battery mechanism by in-situ/operando methods such as in-situ XAS, Raman and SFG spectroscopies as well. He has published over 23 papers on the top journal of Nano Lett., Energy Storage Mater., Adv. Funct. Mater., Adv. Sci, Nano Energy, Energy Environ. Mater., Chem. Eng. J., J. Mater. Chem. A, ChemSusChem, J. Power Sources and ACS Appl. Mater. Interface as the first or corresponding author.
E-mail:jian.wang@kit.edu、wangjian2014@sinano.ac.cn

